
The level and efficacy of lutein in patients with age-related macular degeneration: a comprehensive systematic review and meta-analysis
Introduction
Age-related macular degeneration (AMD), a progressive disorder primarily affecting the central retina and causing irreversible visual impairment (1), is now one of the dominant causes of blindness in elderly people (2). The number of AMD patients worldwide is estimated to be 288 million by 2050 (2). AMD can be further subdivided into neovascular AMD and atrophic AMD according to its pathophysiology. Although intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs has been used as the standard treatment for neovascular AMD (3,4), the need for regular injection, patients’ poor responses to anti-VEGF agents, and the high cost make the use of such treatment schemes relatively limited (5) and there currently are no efficient treatments for atrophic AMD. At present, the main strategies are to use antioxidants, multivitamins, and minerals for treatment, as well as to identify and control risk factors that may increase the incidence and progression of AMD (6).
As the most abundant carotenoid present in the human retina (7), lutein possesses various properties, including oxidation resistance (8), filtering blue light (9), and anti-inflammatory (10). Numerous cellular and animal studies have showed that lutein plays a therapeutic role in alleviating oxidative and inflammatory damages (11-16), which are two major pathological processes in AMD (6). Since lutein must be obtained from the diet (17), some clinical trials exploring the association between dietary supplementation with lutein and AMD have been performed, but the results are inconsistent. Lutein Antioxidant Supplementation Trial (LAST) (18), Lutein Antioxidant Supplementation Trial II (LASTII) (19), Combination of Lutein Effects in the Aging Retina (CLEAR) study (20) and Lutein Intervention Study Austria (LISA) (21) indicated protective effects of lutein for AMD. But Beaver Dam Study (22) and Nurses’ Health Study, Health Professionals Follow-up Study (23,24) failed to show a positive association between lutein intake and AMD. Plasma level of lutein may be another predictive factor shown of AMD risks. Several studies support the possibility that higher levels of antioxidants in the blood, especially carotenoids such as lutein, may be related to the reduced risk of AMD (25,26). Meanwhile, one study showed that there is no difference in blood lutein between AMD patients and controls (27). The inconsistent findings of these studies on the level of lutein in patients with AMD in comparison to healthy controls and the efficacy of lutein intake in AMD patients may be attributable to two main reasons. Firstly, the absorption and metabolism of lutein may be different among people with different age, race and gender. Secondly, the AMD patients in some studies may be included by self-reporting or other different criteria, it may lead to differences in classification of cases and conclusions even with the same interventions. For these questions, meta-analysis maybe a good way to explore the relationship between lutein and AMD by giving the unified inclusion and exclusion criteria and then drawing reliable conclusions.
Although the relationship between lutein and AMD has been explored in some meta-analyses (28-30), which usually involved the combined effect of lutein with other antioxidants, including zeaxanthin, zinc, docosahexaenoic acid and so on. And to the best of our knowledge, there is no meta-analysis to clarify the relationship between lutein in blood and AMD patients.
To clarify these inconsistent findings in the literature and the potential role of lutein in the prevention and progression of AMD, a meta-analysis was performed to evaluate available research on lutein blood levels between AMD patients and controls, and research on lutein supplementation in patients with AMD. We present the following article in accordance with the MOOSE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-173/rc).
Methods
Search strategy
The Cochrane, MEDLINE, Elsevier, PubMed, Web of Knowledge, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biomedical (CBM) databases were electronically searched for publications through April 2020 without language restrictions. The keywords for this search included lutein combined with each of the following words: age-related maculopathy, age-related macular degeneration, and AMD. Additional studies were obtained by manual search of references cited by the screened papers and systematic reviews. The protocol was registered with the INPLASY Register (registration No. INPLASY2021110091).
Selection criteria
The inclusion criteria were as follows: (I) randomized clinical trials (RCT) (studies investigating the effect of lutein supplement on AMD [macular pigment optical density (MPOD) as the outcome] by giving quantitative dietary lutein supplement (test group) and placebo (control group) to AMD patients) and case-control studies (studying the blood lutein level of AMD patients and control subjects) in which the association between lutein and AMD was investigated; (II) studies involving subjects that were >40 years old; (III) AMD was diagnosed by professionals according to specific criteria; (IV) mean, standard deviation, or sufficient data to calculate these were reported; and (V) lutein was supplemented alone and quantified in randomized clinical trials.
Studies with any of the following conditions were excluded: (I) studies involving subjects that were reported to have other eye diseases other than AMD, or received retinal surgery within 3 months, photosensitive drugs, or corticosteroid therapy; (II) repeated reports, poor quality research (not providing enough information for subjects, interventions and research design), and articles where information was not available; (III) abstracts and reviews which could not provide original research data for analysis; and (IV) animal experimental studies.
Literature searches and articles were screened independently by two investigators (W.R. & Y.Z.). An initial screening was performed by examining the titles and abstracts of all the retrieved articles, and the remaining articles were read in full and checked. Discrepancies were resolved by discussion between the two investigators.
Data extraction
After screening the articles, two investigators (L.Y. & N.M.) independently extracted detailed information from the eligible studies. The following data were extracted: the first author’s name, publication date, country where the study was performed, study design, sample size, study population, intervention, AMD diagnostic method, follow-up duration, mean and standard deviation in each subgroup, and outcome measures.
Literature quality assessment
The quality of case-control studies was evaluated independently by two reviewers using the Newcastle Ottawa Scale (NOS), which consists of the following three broad aspects: (I) selection of study groups (four criteria); (II) comparability of study groups (one criterion); (III) assessment of the outcome/exposure (three criteria). Studies that fulfilled all of the criteria were scored 9 stars, and a score ≥6 stars was considered to indicate good quality research. Studies that met four or fewer of these criteria were considered to be either fair or poor quality.
Risk of bias in the RCTs was evaluated using the Cochrane Collaboration tool (31), which is comprised of six aspects: (I) random sequence generation; (II) allocation concealment; (III) blinding of participants and personnel; (IV) blinding of outcome assessment; (V) incomplete outcome data; and (VI) selective reporting. Discrepancies were settled by discussion and consensus.
Statistical analysis
The extracted data were continuous variables, and therefore, mean differences (MDs) with 95% confidence intervals (CIs) were used to summarize and compare between groups. The effects of different doses and treatment durations were compared through subgroup analyses. Between-study heterogeneity was explored by Q tests, with the I2 value quantifying the degree of heterogeneity. If I2>50%, it was considered that the heterogeneity was high, and the random model was applied; otherwise, the fixed effect model was used. Publication bias was assessed by Begg’s test and funnel plots when sufficient studies (n>10) were available. All statistical analyses were conducted by using Stata, version 12.0 (Stata Corporation, College Station, TX, USA). All P values were two-sided, with statistical significance set at a level of 0.05. Sensitivity analysis was performed by excluding one study at a time and assessing whether the pooled results of the remaining studies were different from those of all studies.
Results
In total, 1,520 citations were initially screened, and duplicates, abstracts, case reports, and obviously irrelevant research was excluded. The remaining 28 articles were read in full, and nine studies were finally included in the meta-analysis (Figure 1). Of these studies, five investigated the difference in lutein blood levels between AMD patients and controls, and four explored the relationship between dietary intake of lutein supplement and the risk of AMD.
Study characteristics
Table 1 shows the characteristics of the nine included studies (involving a total of 855 subjects), four of which used a case-control design, one was a dose-ranging study, and four were RCTs.
Table 1
| Study | Country | Study design | Trial duration | Participant characteristics | Intervention | Age (years) | Diagnosis criteria of AMD | Determination method of serum lutein/MPOD |
|---|---|---|---|---|---|---|---|---|
| Studies regarding AMD and blood lutein levels | ||||||||
| Sanders et al. [1993] (27) | UK | Case-control study | – | 65 patients with AMD and 65 control subjects matched for age and sex | – | 66–87 | Defined as having degenerative changes that were clearly visible in both maculas | HPLC |
| Mares-Perlman et al. [1995] (32) | US | Nested case-control study | – | 167 patients with AMD and 167 controls matched with cases for age, sex, and current smoking status. | – | 43–75+ | Wisconsin Age-Related Maculopathy Grading System | HPLC |
| Koh et al. [2004] (33) | Singapore | Case-control study | – | Seven patients with early AMD and six volunteers with no retinal pathology | – | 58–81 | – | HPLC |
| Cardinault et al. [2005] (34) |
France | Case-control study | – | 37 AMD patients and 24 control subjects | – | Control: 71.0±1.5; patients: 74.7±1.2 | Clinical examination by the physician | HPLC |
| Rosenthal et al.* [2006] (35) | USA | Dose-ranging study | – | 45 participants with no AMD (n=15), large drusen (n=15), or advanced AMD (n=15) | – | 60–91 | – | HPLC |
| Studies regarding AMD and MPOD | ||||||||
| Murray et al. [2013] (20) | Netherlands | Randomized, double-blind, placebo-controlled, two-center investigation | 12 months | 73 patients with early AMD | Lutein capsules (10 mg ester) (n=36) or a placebo (n=37) were taken daily | 50–80 | According to the Rotterdam study (36,37) | Flicker-based technique (MPS9000) |
| Huang et al. [2015] (38) | China | Randomized, double-blind, placebo-controlled trial | 2 years | 108 patients with early AMD | 10 mg lutein (n=26); 20 mg lutein (n=27); 10 mg lutein + 10 mg zeaxanthin (n=27); placebo (n=28) daily |
>50 | Age-Related Eye Disease Study System | Fundus autofluorescence images |
| Ma et al. [2017] (39) | China | Randomized, double-blind, placebo-controlled trial | 48 weeks | 200 patients with early AMD | 20 mg lutein (n=100); placebo (n=100) | 50–79 | Fundus photographic severity scale (40) | Not given |
| Gao et al. [2018] (41) | China | Randomized, controlled trial | 3 months | 48 AMD patients; 52 eyes | 20 mg lutein; blank | 61.9±11.24 | Fluorescence fundus angiography and optical coherence tomography | The non-mydriatic fundus camera ZEISS Visucam 500 |
*, this study meets all the inclusion criteria. AMD, age-related macular degeneration; HPLC, high performance liquid chromatography; MPOD, macular pigment optical density.
The four case-control studies and one dose-ranging study assessed blood levels of lutein between AMD patients and controls, comprising a total of 291 patients and 277 controls. Blood levels of lutein were analyzed by high performance liquid chromatography (HPLC) in all of these studies.
The four RCTs investigated the effect of lutein supplementation on MPOD of patients with AMD, which included 429 subjects. Patients in the test group were given quantified dietary lutein supplementation for a period ranging from 3 months to 2 years. Patients in the control group received a placebo. All studies included MPOD as an outcome. The NOS scoring of the case-control studies is displayed in Table 2. The score of all included case-control studies ≥5 stars, which indicates good quality research. For RCTs, two studies (20,36) were rated with a low risk of bias, one study (37) with a moderate risk of bias and one (38) with a high risk of bias according to the Cochrane Collaboration’s tool (31). Figure 2 shows the result of risk of bias assessment.
Table 2
| Study | Adequate definition of cases | Representativeness of cases | Selection of control | Definition of control | Control for important factor or additional facto2 | Exposure assessment | Same method of ascertainment for cases and controls | Non-response rate3 | Total quality scores |
|---|---|---|---|---|---|---|---|---|---|
| Sanders et al. [1993] (27) | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Mares-Perlman et al. [1995] (32) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Koh et al. [2004] (33) | ★ | – | – | ★ | ★ | ★ | ★ | ★ | 6 |
| Cardinault et al. [2005] (34) | – | – | ★ | ★ | ★★ | – | – | ★ | 5 |
| Rosenthal et al. [2006] (35) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
1, a study could be awarded a maximum of one star for each numbered item, except for the item Control for the most important factor or second important factor; 2, a maximum of two stars could be awarded for Control for the most important factor or second important factor. Studies that controlled for other mental and physical illnesses and genetic history received one star, while studies that controlled for age, sex, and living environment received one additional star; 3, one star was awarded if there was no significant difference in the response rate between control subjects and cases in the chi-square test (P>0.05).
Blood lutein levels and AMD
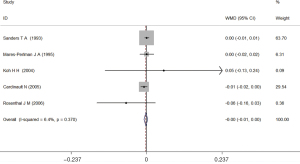
Among the selected five studies, two reported that the blood lutein levels of the cases were higher than those of the controls (32,33), two reported the opposite result (34,35), and one reported no difference in the blood lutein levels between AMD patients and controls (27). There was no significant difference in all results. The fixed-effects meta-analysis of all five studies showed that there was no difference in the total blood lutein between AMD patients and control subjects (WMD =0.00; 95% CI: −0.01 to 0.00; Figure 3).
Effects of lutein supplementation in AMD patients
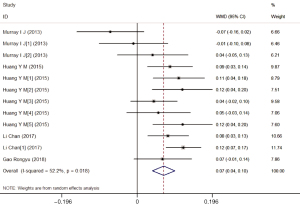
Four studies reported 12 research results according to different doses and durations. Six results identified an association between the lutein intake and a higher content of MPOD, and two studies reported a negative correlation, without statistical significance. There was significant heterogeneity across the studies (I2=52.2%; P for heterogeneity 0.018). The random-effects meta-analysis of all 12 results found a significant increase in MPOD (WMD =0.07; 95% CI: 0.04–0.10) among AMD patients taking lutein supplementation (Figure 4).
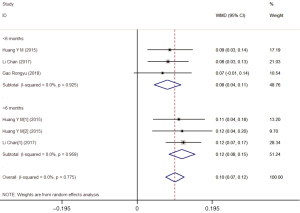
A subgroup analysis (Table 3) according to the dose of lutein supplementation found a significant increase in MPOD only in the treatment subgroup with 20 mg/d (WMD =0.098; 95% CI: 0.074–0.122), but not the treatment group with 10 mg/d lutein (WMD =0.032; 95% CI: −0.018–0.081). Similarly, a subgroup analysis according to the duration of lutein supplementation showed that a significant increase in MPOD only existed in the >6 months group (WMD =0.087; 95% CI: 0.052–0.122), but not in the <6 months group (WMD =0.048; 95% CI: 0.001–0.094).
Table 3
| Subgroup | No. of studies | WMD (95% CI) | P value | Test of heterogeneity | |
|---|---|---|---|---|---|
| P value | I2 | ||||
| Overall | 12 | 0.07 (0.04–0.10) | 0.000 | 0.018 | 52.20% |
| Supplement dose | |||||
| 10 mg | 6 | 0.032 (−0.018–0.081) | 0.047 | 0.206 | 55.50% |
| 20 mg | 6 | 0.098 (0.074–0.122) | 0.133 | 0.000 | 0.00% |
| Supplement duration | |||||
| <6 months | 5 | 0.048 (0.001–0.094) | 0.044 | 0.041 | 59.80% |
| >6 months | 7 | 0.087 (0.052–0.122) | 0.000 | 0.150 | 36.40% |
MPOD, macular pigment optical density; CI, confidence interval; WMD, weighted mean difference.
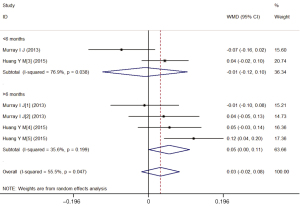
Stratified analyses based on the lutein supplementation dose demonstrated that the levels of MPOD were significantly higher in the treatment group with 20 mg/d lutein compared to the placebo group (WMD =0.10; 95% CI: 0.07–0.12), regardless of whether the duration is more than 6 months (WMD =0.12; 95% CI: 0.08–0.15) or fewer than 6 months (WMD =0.08; 95% CI: 0.04–0.11) (Figure 5). Meanwhile, no difference was observed in the treatment group with 10 mg/d lutein (WMD =0.03; 95% CI: −0.02–0.08) (Figure 6).
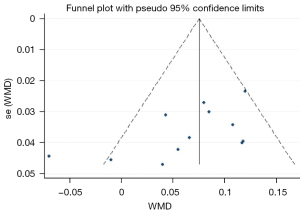
The sensitivity analysis showed that the overall results did not change by excluding any one of studies from the analysis. Also, according to the results of Begg’s test (P=0.086) and the funnel plot (Figure 7), there was no significant publication bias.
Discussion
The results of the present meta-analysis suggested that no difference in blood lutein was observed between the AMD patients and control subjects. However, one meta-analysis (42) showed that the serum lutein level of AMD patients was lower than that of control group, and the difference was statistically significant. The results of that meta-analysis may be doubtful, because among the eight included studies, two determined the levels of total lutein and zeaxanthin, while the remaining six only determined the lutein content, and the original format of the data report in one article did not meet the inclusion requirements.
Data on evaluating the effects of lutein supplementation among AMD patients found that lutein supplement could increase MPOD, which is consistent with the findings of previous meta-analyses (43,44). Stratified analyses by dose and duration indicated that lutein supplement showed efficacy only at high doses (>20 mg) and for long periods of time (>6 months), and there was no significant effect when the dose was 10 mg or the duration was less than 6 months. Feng et al. (43) also reached a consistent result about the duration when evaluating the effects of lutein supplementation on MPOD among AMD patients, but they also showed that dietary intake of lutein at 10 mg/d lasting longer than 6 months can significantly improve MPOD levels in patients with AMD. The divergent result about dose may have come from five studies that involved the combined supplementation of lutein with other antioxidants, including zeaxanthin, docosahexaenoic acid, and eicosapentaenoic acid in Feng et al.’s research (43). Such mixtures may be more effective in antioxidation than individual lutein at the same concentration (45,46). Given that AMD is a chronic progressive disease, this stratified analysis confirmed the importance of dose and duration of use for these supplements. Whether lutein is combined with other antioxidants is also an important factor to determine the effective time and dose. In addition, other factors may have a certain impact on AMD. For example, ethnic (47), increased body fat (48,49), extended exposure to sunlight (50-52), genetic factors (53) and smoking (54) have been shown to play a role in MPOD/AMD. However, the impact of these factors on AMD needs to be confirmed by more studies. In addition to efficacy, safety is also an important factor in supplements. Lutein is classified as Generally Regarded as Safe (GRAS) by the US Food and Drug Administration (FDA) (55). Besides, lutein is safe at the dose up to 20 mg/d according to the Council for Responsible Nutrition (CRN) (56). Two clinical research investigated the effects of lutein with the intake at 40 mg/d for 9 weeks, 20 mg/d thereafter up to 26 weeks and 30 mg/d for 120 days, respectively (57,58). No adverse health effects were reported. Thus, it may be reasonable to supplement lutein at 20 mg/d to improve MPOD in AMD patients for both the safety and efficacy.
There are some weaknesses of the present study that should be noted. Firstly, the study assessing the difference in serum lutein levels between AMD patients and controls was based on observational studies with limited a sample size, which might have potential bias and confounding effects. Secondly, the types and stages of AMD were not strictly distinguished due to the limited information given in the included literature and the various classification criteria applied. Despite these limitations, this study strived to clarify the association between serum lutein levels and AMD. In addition, studies involving the combined supplementation of lutein with other antioxidants were excluded to lower the heterogeneity and increase the reliability of results.
In conclusion, the evidence available to date demonstrates that no difference was observed between AMD patients and control subjects in total serum lutein. Nevertheless, dietary intake of lutein (20 mg/day) can significantly improve MPOD in AMD patients.
Acknowledgments
The authors are thankful for the guidance and support of the West China School of Public Health, Sichuan University.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-173/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-173/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358:2606-17. [Crossref] [PubMed]
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-16. [Crossref] [PubMed]
- Muni RH, Altaweel M, Tennant M, et al. Agreement among Canadian retina specialists in the determination of treatment eligibility for photodynamic therapy in age-related macular degeneration. Retina 2008;28:1421-6. [Crossref] [PubMed]
- Nian S, Lo ACY. Protecting the Aging Retina. Neuroprotection 2019. Available online: https://www.intechopen.com/chapters/64777
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98. [Crossref] [PubMed]
- Li LH, Lee JC, Leung HH, et al. Lutein Supplementation for Eye Diseases. Nutrients 2020;12:1721. [Crossref] [PubMed]
- Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 2014;72:605-12. [Crossref] [PubMed]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459-516. [Crossref] [PubMed]
- Junghans A, Sies H, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys 2001;391:160-4. [Crossref] [PubMed]
- Kijlstra A, Tian Y, Kelly ER, et al. Lutein: more than just a filter for blue light. Prog Retin Eye Res 2012;31:303-15. [Crossref] [PubMed]
- Chae SY, Park SY, Park G. Lutein protects human retinal pigment epithelial cells from oxidative stress-induced cellular senescence. Mol Med Rep 2018;18:5182-90. [Crossref] [PubMed]
- Fernández-Robredo P, Recalde S, Arnáiz G, et al. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-E deficient mice. Curr Eye Res 2009;34:543-52. [Crossref] [PubMed]
- Fernández-Robredo P, Sádaba LM, Salinas-Alamán A, et al. Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse. Oxid Med Cell Longev 2013;2013:213505. [Crossref] [PubMed]
- Age-Related Eye Disease Study Research Group; SanGiovanni JP, Chew EY, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007;125:1225-32.
- Madhavan J, Chandrasekharan S, Priya MK, et al. Modulatory Effect of Carotenoid Supplement Constituting Lutein and Zeaxanthin (10:1) on Anti-oxidant Enzymes and Macular Pigments Level in Rats. Pharmacogn Mag 2018;14:268-74. [Crossref] [PubMed]
- Ramkumar HL, Tuo J, Shen DF, et al. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. J Nutr 2013;143:1129-35. [Crossref] [PubMed]
- Chung RWS, Leanderson P, Gustafsson N, et al. Liberation of lutein from spinach: Effects of heating time, microwave-reheating and liquefaction. Food Chem 2019;277:573-8. [Crossref] [PubMed]
- Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-30. [Crossref] [PubMed]
- Richer S, Devenport J, Lang JC. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry 2007;78:213-9. [Crossref] [PubMed]
- Murray IJ, Makridaki M, van der Veen RL, et al. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci 2013;54:1781-8. [Crossref] [PubMed]
- Weigert G, Kaya S, Pemp B, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:8174-8. [Crossref] [PubMed]
- VandenLangenberg GM, Mares-Perlman JA, Klein R, et al. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol 1998;148:204-14. [Crossref] [PubMed]
- Cho E, Hankinson SE, Rosner B, et al. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87:1837-43. [Crossref] [PubMed]
- Cho E, Seddon JM, Rosner B, et al. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol 2004;122:883-92. [Crossref] [PubMed]
- Delcourt C, Carrière I, Delage M, et al. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci 2006;47:2329-35. [Crossref] [PubMed]
- Antioxidant status and neovascular age-related macular degeneration. Eye Disease Case-Control Study Group. Arch Ophthalmol 1993;111:104-9. [Crossref] [PubMed]
- Sanders TA, Haines AP, Wormald R, et al. Essential fatty acids, plasma cholesterol, and fat-soluble vitamins in subjects with age-related maculopathy and matched control subjects. Am J Clin Nutr 1993;57:428-33. [Crossref] [PubMed]
- Ma L, Dou HL, Wu YQ, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr 2012;107:350-9. [Crossref] [PubMed]
- Chong EW, Wong TY, Kreis AJ, et al. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ 2007;335:755. [Crossref] [PubMed]
- Lem DW, Davey PG, Gierhart DL, et al. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants (Basel) 2021;10:1255. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Mares-Perlman JA, Brady WE, Klein R, et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol 1995;113:1518-23. [Crossref] [PubMed]
- Koh HH, Murray IJ, Nolan D, et al. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res 2004;79:21-7. [Crossref] [PubMed]
- Cardinault N, Abalain JH, Sairafi B, et al. Lycopene but not lutein nor zeaxanthin decreases in serum and lipoproteins in age-related macular degeneration patients. Clin Chim Acta 2005;357:34-42. [Crossref] [PubMed]
- Rosenthal JM, Kim J, de Monasterio F, et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest Ophthalmol Vis Sci 2006;47:5227-33. [Crossref] [PubMed]
- Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol 1998;116:653-8. [Crossref] [PubMed]
- van Leeuwen R, Ikram MK, Vingerling JR, et al. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-7. [Crossref] [PubMed]
- Huang YM, Dou HL, Huang FF, et al. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: a randomised, double-blind, placebo-controlled trial. Br J Ophthalmol 2015;99:371-5. [Crossref] [PubMed]
- Ma L, Yan SF, Huang YM, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012;119:2290-7. [Crossref] [PubMed]
- Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484-98. [Crossref] [PubMed]
- Gao RY, Zhang J, Sun XY, et al. Clinical observation of macular pigment optical density changes on visual functions in patients with age-related macular degeneration. Journal of Binzhou Medical University 2018;41:185-8.
- Dong Y, Zhang FD, Wang T, et al. Meta-analysis of the relationship between serum lutein level and age-related macular degeneration. Chinese Preventive Medicine 2012;13:492-6.
- Feng L, Nie K, Jiang H, et al. Effects of lutein supplementation in age-related macular degeneration. PLoS One 2019;14:e0227048. [Crossref] [PubMed]
- Wang X, Jiang C, Zhang Y, et al. Role of lutein supplementation in the management of age-related macular degeneration: meta-analysis of randomized controlled trials. Ophthalmic Res 2014;52:198-205. [Crossref] [PubMed]
- Meagher KA, Thurnham DI, Beatty S, et al. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br J Nutr 2013;110:289-300. [Crossref] [PubMed]
- Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys 2010;504:56-60. [Crossref] [PubMed]
- Wolf-Schnurrbusch UE, Röösli N, Weyermann E, et al. Ethnic differences in macular pigment density and distribution. Invest Ophthalmol Vis Sci 2007;48:3783-7. [Crossref] [PubMed]
- Hammond BR Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci 2002;43:47-50. [PubMed]
- Nolan JM, Stack J, O' Donovan O, et al. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res 2007;84:61-74. [Crossref] [PubMed]
- West SK, Rosenthal FS, Bressler NM, et al. Exposure to sunlight and other risk factors for age-related macular degeneration. Arch Ophthalmol 1989;107:875-9. [Crossref] [PubMed]
- Taylor HR, West S, Muñoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol 1992;110:99-104. [Crossref] [PubMed]
- Darzins P, Mitchell P, Heller RF. Sun exposure and age-related macular degeneration. An Australian case-control study. Ophthalmology 1997;104:770-6. [Crossref] [PubMed]
- Liew SH, Gilbert CE, Spector TD, et al. Macular Pigment Heritability: A Twin Study. Invest Ophthalmol Vis Sci 2005;46:4430-6. [Crossref] [PubMed]
- McCarty CA, Mukesh BN, Fu C. Risk Factors for Age-Related Maculopathy: The Visual Impairment Project. Arch Ophthalmol 2001;119:1455. [Crossref] [PubMed]
- Ranard KM, Jeon S, Mohn ES, et al. Dietary guidance for lutein: consideration for intake recommendations is scientifically supported. Eur J Nutr 2017;56:37-42. [Crossref] [PubMed]
- Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol 2006;45:289-98. [Crossref] [PubMed]
- Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry 2000;71:147-64. [PubMed]
- Wenzel AJ, Sheehan JP, Gerweck C, et al. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol Opt 2007;27:329-35. [Crossref] [PubMed]
(English Language Editor: A. Kassem)




