
CD73+ adipose-derived stem cells reduce scar formation through PLOD1
Introduction
Damaged cutaneous tissue can be replaced with an abnormal connective tissue during tissue regeneration to generate a specific structure known as a scar (1). The formation of scar impairs the regulator functionality of the normal skin, resulting in reduction in tensile and dysfunction (2). Anti-inflammatory therapy with IL-10 or tumor necrosis factor alpha stimulated gene 6, or anti-angiogenesis therapy with anti-vascular endothelial growth factor monoclonal antibody may help to inhibit scar formation.
Stem cell transplantation has been shown to be an effective treatment during wound healing (3), in which scar formation is substantially reduced. Mesenchymal stem cells (MSCs), especially adipose-derived MSCs (AMSCs), are most used due to their relative safety, easy access, and rich abundance (4-7). We recently showed that transcriptional regulation of procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1 (PLOD1) expression in AMSCs can further improve the therapeutic outcome of AMSCs. PLOD1 has been shown to be crucial for lysine residue hydroxylation in collagen telopeptides and for the collagen pyridinoline to develop fibrotic cross-links (8). Previous studies have demonstrated a beneficial effect of PLOD1 suppression on skin regeneration with reduced scar formation (9-14). However, to the best of our knowledge, our study is the first to show that the therapeutic potential of AMSCs can be improved by modulation of PLOD1 levels.
CD73, encoded by the NT5E gene in humans, is an enzyme that mediates conversion of adenosine monophosphate to adenosine (15). Recent studies have shown that CD73 can be used as a marker to separate AMSCs into the following two subgroups: CD73+ AMSCs and CD73– AMSCs. CD73+ AMSCs demonstrate better effects during cardiac muscular regeneration and during recovery from spinal cord injury (16,17). However, whether CD73+ AMSCs are more beneficial than total AMSCs or CD73– AMSCs in skin wound repair, as well as the role of PLOD1 in CD73+ AMSCs in terms of regenerative potential, is not known, and therefore, addressed in this study.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6557/rc).
Methods
Protocols and animals
The present study received approval from the Research and Animal Ethics Association of Shanghai Jiao Tong University (No. XHEC-F-2021-071) in compliance with Shanghai Jiao Tong University institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration. Both male and female 12-week-old C57BL/6 mice (about 25 and 20 g, respectively) were used in the present study, and were both purchased from Shanghai Laboratory Animals Center Laboratory Animal Co., Ltd. (Shanghai, China). Mice of different sexes were distributed evenly into each experimental group. A 12-h light-dark cycle was applied to mouse housing. The mouse number in each experimental group was determined by power calculations (P<0.05) to guarantee the legitimacy of the results. An allocation concealment method was applied to reduce confounders. No criteria were used for excluding animals during the experiment. No data were excluded during the analysis. No humane endpoints were involved in the current study. Skin injury was induced as previously described. For the in vivo experiment, the mice were allocated to the following 9 groups: group 1, mice were sham treated; group 2, mice received skin injury and transplantation of saline; group 3, mice received skin injury and transplantation of AMSCs; group 4, mice received skin injury and transplantation of CD73– AMSCs; group 5, mice received skin injury and transplantation of CD73– AMSCs transfected with scrambled (scr) plasmid; group 6, mice received skin injury and transplantation of CD73– AMSCs transfected with siRNA for PLOD1 (siPLOD1) plasmid; group 7, mice received skin injury and transplantation of CD73+ AMSCs; group 8, mice received skin injury and transplantation of CD73+ AMSCs transfected with scr; and group 9, mice received skin injury and transplantation of CD73+ AMSCs transfected with PLOD1 plasmid.
Isolation and sorting for CD73– versus CD73+ AMSCs
AMSCs were isolated from 12-week-old C57BL/6 mice, as previously described. AMSCs were cultured in Dulbecco’s modified Eagle medium supplemented with 15% fetal bovine serum. For separation of CD73– versus CD73+ AMSCs, a PE-cy7-conjugated rabbit anti-mouse CD73 antibody was used for flow cytometry-based cell soring. Data were analyzed using FlowJo software (FlowJo LLC., Ashland, OR, USA).
Transfection of AMSCs
Transfection of AMSCs was done with Lipofectamine 3000 reagent (Invitrogen, St Louis, MO, USA) using plasmids carrying complete coding sequence for PLOD1, or a siRNA for PLOD1 (siPLOD1), or a scr as a control, all under control of a cytomegalovirus (CMV) promoter. The construct also contained a green fluorescent protein reporter (connected with a 2A sequence) with the transgene to allow evaluation of the transfection efficiency (about 90% in total).
Transplantation of different AMSCs
A total of 2×106 differently modified AMSCs were prepared in 150 µL normal saline and then injected into the tail vein of the mice 3 days before injury formation, as the transplanted cells need time for homing.
Histology
Masson trichrome staining was performed using trichrome Stain (Masson) kit (Sigma-Aldrich, St. Louis, MO, USA).
Enzyme-linked immunosorbent assay (ELISA)
Protein levels for PLOD1 and CD73 were detected by anti-PLOD1 or anti-CD73 ELISA kits (R&D, Carpinteria, CA, USA), respectively.
Quantitative polymerase chain reaction
Total RNA extraction was done using an RNeasy kit (Qiagen, Hilden, Germany), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed with a QuantiTect SYBR Green PCR kit (Qiagen, Shanghai, China). Commercial primers were all purchased from Qiagen. An 2−ΔΔCt method was used for analysis of gene transcript levels. Relative gene expression was determined by sequential normalization of the values against internal and experimental controls.
Bioinformatics and statistical analysis
A bioinformatics analysis on the differential gene profiles of CD73– versus CD73+ AMSCs was done using a validated public dataset, GSE167219. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data quality was carefully confirmed using mean difference plot, mean variance trend plot, t-statistic quantile-quantile plot, and boxplot to ensure that all of the included data were reliable. All statistical analyses (one-way analysis of variance with a Bonferroni correction) were conducted by GraphPad Prism software (version 7; GraphPad Software, La Jolla, CA, USA). Data were represented as mean ± standard deviation and the significance was set at P<0.05.
Results
Validation of a gene profile data for CD73– versus CD73+ AMSCs
Recent studies have shown that CD73 can be used as a marker to separate AMSCs into the following two subgroups: CD73+ AMSCs and CD73– AMSCs. CD73+ AMSCs demonstrate better effects during cardiac muscular regeneration and during recovery from spinal cord injury (16,17). However, whether CD73+ AMSCs are more beneficial than total AMSCs or CD73– AMSCs in skin wound repair, as well as the role of PLOD1 in the CD73+ AMSCs in terms of regenerative potential, is not known. Therefore, we first examined public databases to address these questions.
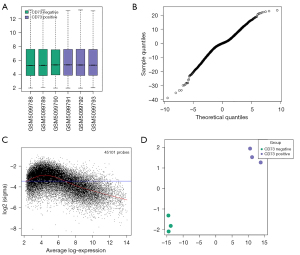
A public dataset (GSE167219) was validated by boxplot analysis (Figure 1A), mean variance trend plot (Figure 1B), t-statistic quantile-quantile plot (Figure 1C), and principal component analysis (PCA) plot (Figure 1D) to ensure that the data in this database were reliable and for analysis.
PLOD1 levels are significantly higher in CD73– AMSCs versus CD73+ AMSCs
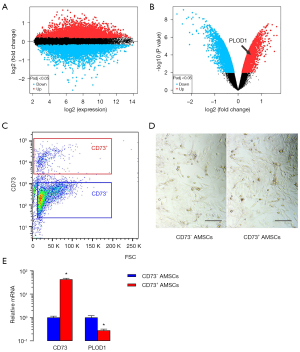
We then examined the differential genes between CD73– AMSCs and CD73+ AMSCs. The results are shown in volcano maps (Figure 2A,2B). We found that PLOD1 levels are significantly higher in CD73– AMSCs versus CD73+ AMSCs (Figure 2B). To further confirm it, we separated CD73– AMSCs versus CD73+ AMSCs by flow cytometry (Figure 2C). The isolated CD73– AMSCs and CD73+ AMSCs did not show big difference in morphology in culture (Figure 2D). qRT-PCR for CD73 was performed on CD73– AMSCs versus CD73+ AMSCs, which confirmed the proper separation of populations based onCD73 levels.PLOD1 levels in CD73+ AMSCs were lower than those CD73– AMSCs by more than 80% (Figure 2E).
Preparation of plasmids to modify PLOD1 levels in CD73– AMSCs and CD73+ AMSCs
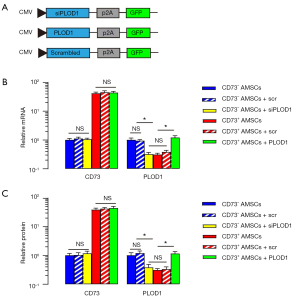
After detecting significantly lower PLOD1 in CD73+ AMSCs versus CD73– AMSCs, and demonstrating the beneficial effect of AMSCs by PLOD1 depletion, we hypothesized that CD73+ AMSCs might have a better effect on wound repair and generate reduced scar formation compared with total AMSCs or CD73– AMSCs. To prove this, we first generated plasmids to modify PLOD1 levels through expressing PLOD1 or siPLOD1. Plasmids carrying a scr sequence were used as a control (Figure 3A). We first confirmed the CD73 mRNA levels (Figure 3B) and CD73 protein levels (Figure 3C) in CD73+ AMSCs versus CD73– AMSCs by qRT-PCR and ELISA, respectively. Neither the expression of PLOD1 nor siPLOD1 altered levels of CD73 (Figure 3B,3C). Moreover, siPLOD1 significantly decreased PLOD1 mRNA levels (Figure 3B) and PLOD1 protein levels (Figure 3C) in CD73– AMSCs, while PLOD1 significantly increased PLOD1 mRNA levels (Figure 3B) and PLOD1 protein levels (Figure 3C) in CD73+ AMSCs.
Transplantation of CD73+ AMSCs further reduces fibrosis after skin injury in mice compared with transplantation of total or CD73– AMSCs
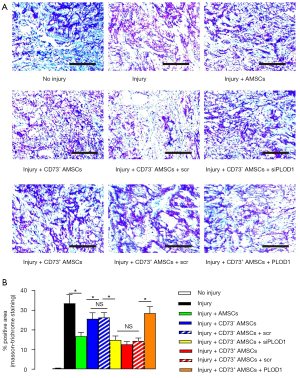
The in vivo effects of the specific AMSC subpopulations on post-injury fibrosis on mouse skin were then analyzed. The mice were assigned to the following nine groups: group 1, mice were sham treated; group 2, mice received skin injury and transplantation of saline; group 3, mice received skin injury and transplantation of AMSCs; group 4, mice received skin injury and transplantation of CD73– AMSCs; group 5, mice received skin injury and transplantation of CD73– AMSCs transfected with scr plasmid; group 6, mice received skin injury and transplantation of CD73– AMSCs transfected with siPLOD1 plasmid; group 7, mice received skin injury and transplantation of CD73+ AMSCs; group 8, mice received skin injury and transplantation of CD73+ AMSCs transfected with scr plasmid; and group 9, mice received skin injury and transplantation of CD73+ AMSCs transfected with PLOD1 plasmid. We evaluated the effects on fibrosis 2 weeks after skin injury. Through quantitative analysis on fibrotic tissue by Masson staining, we found that transplantation of CD73+ AMSCs resulted in less fibrosis on injured mouse skin compared with transplantation of total AMSCs, while transplantation of CD73– AMSCs resulted in more fibrosis on injured mouse skin compared with transplantation of total AMSCs (Figure 4A,4B), suggesting that CD73+ AMSCs could have a better therapeutic potential.
Better therapeutic effects of CD73+ AMSCs on skin wound repair could result from reduced PLOD1 expression
Next, we found that PLOD1 depletion completely abolished increased fibrosis by CD73– AMSC transplantation, while no difference was detected between transplantation of CD73– AMSCs and transplantation of CD73– AMSCs transfected with scr (Figure 4A,4B). Moreover, PLOD1 expression completely abolished reduced fibrosis by CD73+ AMSC transplantation, while no difference was detected between transplantation of CD73+ AMSCs and transplantation of CD73+ AMSCs transfected with scr (Figure 4A,4B). Together, these data suggest that better therapeutic effects of CD73+ AMSCs on skin wound repair could result from reduced PLOD1 expression.
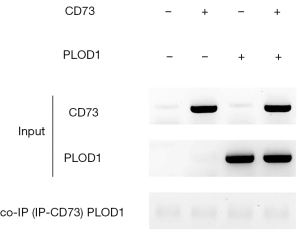
CD73 does not directly interact with PLOD1
Finally, we examined whether CD73 might directly interact with PLOD1. Co-immunoprecipitation for CD73 and PLOD1 was then performed, showing no direct interaction between them (Figure 5). Therefore, CD73 could regulate PLOD1 indirectly.
Discussion
It is very important to generate a more effective stem cell-based therapeutic approach to boost skin wound repair and to determine the underlying cellular and molecular mechanisms (5-7). PLOD1 appeared to be a rising star at the center of the studies, and our recent studies specifically used PLOD1 as a potential target.
Recently, we showed that PLOD1 can be post-transcriptionally suppressed in AMSCs, which improves the outcome of AMSC transplantation for skin wound repair. Differential ability of AMSC has a limitation. However, in the present study, we further showed that CD73+ AMSCs have better therapeutic potential compared with total AMSCs, likely due to the low expression of PLOD1. It is noteworthy that previous studies have demonstrated that CD73+ AMSCs are more like stem cells and have a regulatory effect on inflammation, perhaps through recruitment and polarization of macrophages (16). We have previously shown that the profibrotic effect of PLOD1 could result from its regulation of macrophage polarization. Therefore, the effects of CD73+ AMSCs on macrophages could at least partially result from their low expression of PLOD1.
The cross-talk between PLOD1 and CD73 is unknown. Several online protein interaction tools have been examined; none showed a direct binding relationship between the two proteins. Our data also showed no evidence of direct interaction between PLOD1 and CD73; therefore, their regulatory relationship should be indirect and could involve multiple signaling pathways.
In the present study, we did not analyze the recruitment, proliferation, and polarization of macrophages by CD73+ AMSCs compared with total AMSCs and CD73– AMSCs. These should be investigated in future studies, as macrophages are likely important targets for both CD73 and PLOD1. It might also be interesting to compare the wound healing effect of CD73+ AMSCs and adipose stem cells exosomes. Other potential mechanisms of CD73+ AMSCs to inhibit scar formation may involve decreases in nitric oxide, p53 and TGFβ activity to inhibit fibroblast proliferation.
In conclusion, the current study, we provided robust evidence for using CD73+ AMSCs as a novel therapeutic approach to boost skin wound repair with attenuated scar formation.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81701949).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6557/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6557/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6557/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Research and Animal Ethics Association of Shanghai Jiao Tong University (No. XHEC-F-2021-071) in accordance with Shanghai Jiao Tong University institutional guidelines for the care and use of animals. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Visscher MO, Bailey JK, Hom DB. Scar treatment variations by skin type. Facial Plast Surg Clin North Am 2014;22:453-62. [Crossref] [PubMed]
- Rhett JM, Ghatnekar GS, Palatinus JA, et al. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol 2008;26:173-80. [Crossref] [PubMed]
- Li Q, Zhang C, Fu X. Will stem cells bring hope to pathological skin scar treatment? Cytotherapy 2016;18:943-56. [Crossref] [PubMed]
- Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther 2012;3:20. [Crossref] [PubMed]
- Wang Y, Chu Y, Yue B, et al. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget 2017;8:23803-16. [Crossref] [PubMed]
- Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016;7:74537-56. [Crossref] [PubMed]
- Zhong Z, Gu H, Peng J, et al. GDNF secreted from adipose-derived stem cells stimulates VEGF-independent angiogenesis. Oncotarget 2016;7:36829-41. [Crossref] [PubMed]
- Anum EA, Hill LD, Pandya A, et al. Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta 2009;30:207-15. [Crossref] [PubMed]
- Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015;43:803-16. [Crossref] [PubMed]
- van den Bogaerdt AJ, van der Veen VC, van Zuijlen PP, et al. Collagen cross-linking by adipose-derived mesenchymal stromal cells and scar-derived mesenchymal cells: Are mesenchymal stromal cells involved in scar formation? Wound Repair Regen 2009;17:548-58. [Crossref] [PubMed]
- Ulrich MM, Verkerk M, Reijnen L, et al. Expression profile of proteins involved in scar formation in the healing process of full-thickness excisional wounds in the porcine model. Wound Repair Regen 2007;15:482-90. [Crossref] [PubMed]
- van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol 2004;23:251-7. [Crossref] [PubMed]
- Bank RA, Robins SP, Wijmenga C, et al. Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci U S A 1999;96:1054-8. [Crossref] [PubMed]
- Yamada H, Tajima S, Nishikawa T, et al. Tranilast, a selective inhibitor of collagen synthesis in human skin fibroblasts. J Biochem 1994;116:892-7. [Crossref] [PubMed]
- Alcedo KP, Bowser JL, Snider NT. The elegant complexity of mammalian ecto-5'-nucleotidase (CD73). Trends Cell Biol 2021;31:829-42. [Crossref] [PubMed]
- Li Q, Hou H, Li M, et al. CD73+ Mesenchymal Stem Cells Ameliorate Myocardial Infarction by Promoting Angiogenesis. Front Cell Dev Biol 2021;9:637239. [Crossref] [PubMed]
- Zhai X, Chen K, Yang H, et al. Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury. J Nanobiotechnology 2021;19:274. [Crossref] [PubMed]
(English Language Editor: R. Scott)


