
Clinical manifestations and acid alpha-glucosidase mutation characterisation of a cohort of patients with late-onset Pompe disease in eastern China
Introduction
Pompe disease [glycogen storage disease type II (GSD II)] is a rare, progressive, and life-threatening autosomal recessive disorder caused by the lysosomal enzyme acid alpha-glucosidase (GAA) being significantly reduced or missing (1). Depending on their age at symptom onset, patients are typically classified as infantile-onset Pompe disease (IOPD) or late-onset Pompe disease (LOPD) (2,3).
In its infantile-onset form, which is characterised by predominant and severe cardiomyopathy, the disease has a high mortality rate. The symptoms of LOPD are less severe than those of IOPD, and mainly include progressive proximal limb and respiratory muscle weakness, with cardiomyopathy being uncommon (4). Due to its non-specific symptoms, LOPD is difficult to differentiate from other neuromuscular diseases. Muscle biopsy can detect glycogen deposition by periodic acid-Schiff staining. Decreased GAA activity in lymphocytes and GAA gene mutations are also important for the diagnosis of LOPD.
The GAA gene is located on chromosome 17q25.2-25.3 and contains 20 exons (5). As of August 2021, almost 900 different variants had been listed in the Pompe Disease Mutation Database. These gene mutations exhibit race specificity. In Caucasian and Belgian patients, for example, the most common mutation is c.-32-13T>G (6), whereas c.1935C>A (p.D645E) and c.2238G>C (p.W746C) frequently appear in Taiwanese patients (7). However, despite the increasing awareness of Pompe disease, data on LOPD in patients from mainland China are still limited.
In this study, the clinical features of 14 mainland Chinese patients with LOPD were examined, and genetic analysis was performed. The following article is presented in accordance with the Materials Design Analysis Reporting (MDAR) reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3710).
Methods
Patients
This study included 14 patients (Table 1) who were diagnosed with LOPD in the First Affiliated Hospital of Nanjing Medical University between 2017 and 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Review Board of The First Affiliated Hospital of Nanjing Medical University (2021-SR-415). The patients and their families gave informed consent for the use of their medical data for research purposes.
Table 1
| No. | Gender | Initial symptoms | Age (years) | CK (U/L) | GAA activity (μmol/L/h) | Nucleotide change | Requirement of ventilator support | ||
|---|---|---|---|---|---|---|---|---|---|
| Onset | Diagnosis | Allele1 | Allele2 | ||||||
| 1 | M | Chest congestion, asthma | 20 | 20 | 1,153 | 0.23 | c.2161G>T | c.2238G>C | No |
| 2 | M | Airway infections, respiratory failure | 36 | 36 | 300 | 0.30 | c.1409A>G# | c.1409A>G# | Yes |
| 3 | M | Scoliosis | 16 | 16 | 369 | 0.17 | c.1935C>A | c.2238G>C | No |
| 4 | M | Muscle weakness of limbs | 11 | 16 | 2,336 | – | c.784G>A | c.2297A>G | No |
| 5 | F | Muscle weakness of limbs | 8 | 8 | 1,969 | 0.18 | c.1129G>A | c.2154delC | Yes |
| 6 | F | Muscle weakness of limbs | 12 | 23 | 387 | 0.16 | c.1320_1322delGAT | c.2238G>C | Yes |
| 7 | F | Muscle weakness of limbs | 16 | 23 | 837 | 0.00 | c.1409A>G# | c.2161G>T | Yes |
| 8 | F | Muscle weakness of limbs | 17 | 17 | 501 | 0.19 | c.1634C>T | c.1822C>T | Yes |
| 9 | F | Muscle weakness of limbs | 7 | 29 | 530 | 0.24 | c.1634C>T | c.2242delG# | Yes |
| 10 | M | Muscle weakness of limbs | 12 | 18 | 950 | 0.20 | c.2237G>A | c.2238G>C | No |
| 11 | M | Airway infections, respiratory failure | 14 | 31 | 1,018 | 0.13 | c.1299G>C# | c.2832delA# | Yes |
| 12 | M | Scoliosis | 14 | 15 | 660 | 0.14 | c.1299G>C# | c.2832delA# | No |
| 13 | F | Airway infections, respiratory failure | 27 | 27 | 556 | 0.25 | c.1879_1881delTCC | c.2297A>G | Yes |
| 14 | F | Muscle weakness of limbs | 39 | 47 | 229 | 0.36 | c.1634C>T | c.2426delC | No |
The reference range for GAA enzyme activity is 1.46–20.34 μmol/L/h. #, the novel variants. CK, creatine kinase; GAA, acid alpha-glucosidase; M, male; F, female.
Medical records of clinical manifestations and laboratory results were available for all confirmed cases. According to Chinese diagnostic guidelines, diagnoses were confirmed based on clinical features, reduced GAA enzymatic activity, and mutations in the GAA gene. Dual-energy X-ray absorptiometry was used to test bone mineral density, and whole-spine radiographs were performed to check for scoliosis. Respiratory conditions were assessed by pulmonary function test; however, some patients were unable to cooperate with the pulmonary function test due to their extremely poor respiratory function.
Measurements of GAA enzymatic activity
The NeoLSD MSMS kit (Perkin Elmer, USA) was used for quantitative measurement of GAA enzymatic activity in dried blood spots. A 3.2-mm (1/8 inch) dried blood spot was incubated in buffer solution containing the internal standard and substrate supplied with the kit, at 37 °C for 18.5 hours. Following this, water and the NeoLSD extraction solution were added for extraction. Fifty microliters of supernatant was transferred to a new well and dried with nitrogen. One hundred microliters of flow solvent was added to the well before the experiment was run on the Waters UPLC/Xevo TQD tandem mass spectrometer. Data were obtained by using the multiple reaction monitoring mode of the tandem mass spectrometry system. Enzymatic activity was measured according to the signal response of the target analyte relative to the internal standard, and was expressed as µmol/L/h. The reference range for GAA enzymatic activity is 1.46–20.34 µmol/L/h.
Next-generation sequencing of the GAA gene
Genomic DNA was extracted from peripheral blood samples of 14 patients using the CWE9600 Blood DNA Kit (CW2531, CWBio, Beijing, China). The GAA genes were amplified using specific primers. Following this, the DNA concentrations were determined using the Qubit dsDNA HS Assay Kit (Q32851, Invitrogen, Carlsbda, CA, USA) with a Qubit fluorometer (Q33216, Invitrogen). Quality control was carried out through agarose gel (1%) electrophoresis. Finally, 1 µg of amplified DNA per sample was collected for the generation of a sequencing library. Quantified DNA samples were sheared into 200–300 bp fragments. The KAPA Library Preparation Kit (KK8234, Kapa Biosystems, Boston, MA, USA) was used to prepare DNA libraries in adherence with the manufacturer’s instructions. The constructed libraries were sequenced on the Illumina HiSeq X10 platform (Illumina, San Diego, USA) using a 150-bp paired-end reads protocol.
The raw data (stored in the FASTQ format) obtained from HiSeq X10 was subjected to quality control and aligned to the human reference genome sequence hg19 (http://genome.ucsc.edu/) using Burrows-Wheeler Aligner (http://bio-bwa.sourceforge.net/). The GATK (Genome Analysis ToolKit) software (http://www.broadinstitute.org/gatk) was used to call single-nucleotide polymorphisms. The called variants were annotated using several public databases including 1000 Genomes Project, ExAC, gnomAD, ESP6500, CCDS, RefSeq, and Ensembl. Annotation content included the variant position, variant type, allele frequency, and conservation prediction would help to locate mutations related to LOPD.
All GAA variants discovered by next-generation sequencing were confirmed by Sanger sequencing using specific primers. Twenty micromoles of quantified DNA was added into the polymerase chain reaction (PCR) mixture, which contained 12.5 µL 2× Phanta Max Buffer, 0.5 µL Phanta Max Super-Fidelity DNA Polymerase (1 U/µL), 0.5 µL dNTP Mix (10 mM each), 1 µL forward primers, 1 µL reverse primers, and water, to a final volume of 25 µL. The following protocol was used for amplification: denaturation at 95 °C for 5 minutes, followed by 25 cycles (95 °C for 30 seconds, 65 °C for 30 seconds, decreasing by 0.6 °C per cycle, and then 72 °C for 30 seconds) and 20 cycles (95 °C for 30 seconds, 50 °C for 30 seconds, and then 72 °C for 30 seconds), and then 72 °C for 10 minutes as a final elongation step. Pedigree verification was also used to confirm the variants detected in the patients.
Results
Clinical symptoms of the patients
The 14 patients came from eastern China and included two brothers (No. 11 and 12) from the same family. Table 1 summarises the clinical manifestations of the 14 patients. The male-to-female ratio was 1:1. The median ages at symptom onset and diagnosis were 15.0 years (7–36 years) and 21.5 years (8–47 years), respectively. The median diagnostic delay from onset was 3.0 years (0–22 years).
Eight patients (57.1%, 8/14) displayed a progressive limb weakness as an initial symptom. For three patients (21.4%, 3/14), airway infection with respiratory failure was the first symptom. Two patients (No. 3 and 12) presented with scoliosis as the first symptom and subsequently underwent surgery. One patient’s initial symptoms included chest congestion and onset of asthma.
Although the patients in the cohort presented without hypertrophic cardiomyopathy, patients 1 and 13 exhibited mild mitral and tricuspid insufficiency; further, patient 13 displayed extrapulmonary arterial hypertension. Figure 1 and Table 2 show the clinical features of the patients. All patients had different degrees of limb-gird muscle weakness and osteoporosis during the course of disease progression. A large proportion of the patients (71.43%, 10/14) had gastrointestinal dysfunction and presented with intermittent diarrhoea. Due to respiratory muscle (diaphragm and chest wall) and laryngopharyngeal muscle weakness, some patients demonstrated dyspnoea (57.14%, 8/14), sleep apnoea and sleep-disordered breathing (57.14%, 8/14), bucking and dysphagia (35.71%, 5/14), and diaphragmatic spasm (14.29%, 2/14). None of our cohort had cognitive impairment.
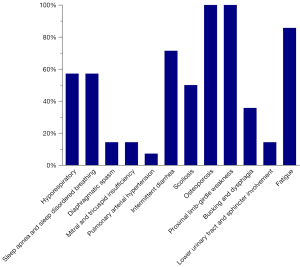
Table 2
| Clinical manifestation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory system | ||||||||||||||
| Dyspnea | + | + | + | + | + | + | + | + | ||||||
| Sleep apnea and sleep disordered breathing | + | + | + | + | + | + | + | + | ||||||
| Diaphragmatic spasm | + | + | ||||||||||||
| Cardiac system | ||||||||||||||
| Mitral and tricuspid insufficiency | + | + | ||||||||||||
| Pulmonary arterial hypertension | + | |||||||||||||
| Gastrointestinal system | ||||||||||||||
| Intermittent diarrhea | + | + | + | + | + | + | + | + | + | + | ||||
| Skeletal system | ||||||||||||||
| Scoliosis | + | + | + | + | + | + | + | |||||||
| Osteoporosis | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Muscular system | ||||||||||||||
| Proximal limb-girdle weakness | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Bucking and dysphagia | + | + | + | + | + | |||||||||
| Urinary system | ||||||||||||||
| Lower urinary tract and sphincter involvement | + | + | ||||||||||||
| Fatigue | + | + | + | + | + | + | + | + | + | + | + | + |
“+” represents the patient has corresponding symptom. GAA, acid alpha-glucosidase.
GAA activity was detected in 13 patients, and the mean level was 0.20 µmol/L/h (0–0.36 µmol/L/h). Two patients (No. 4 and 7) received non-regular and insufficient amounts of enzyme replacement therapy (ERT); recent retesting showed GAA activity of 30.54 and 2.02 µmol/L/h, respectively. The mean serum creatine kinase level at diagnosis was 842.5 U/L (300–2,336 U/L).
Genotypes of patients
Next-generation sequencing of the GAA gene was performed in all 14 patients. In total, 17 GAA mutations, including 11 missense types and 6 deletion types (Figure 2, Table S1), were detected in this study. The results are summarised in Table 1 and show that c.2238G>C (p.W746C) was the most frequent variant, being found at an allele frequency of 14.3% (4/28). The second most frequent variants were c.1409A>G (p.N470S) and c.1634C>T (p.P545L), with an allele frequency of 10.7% (3/28). According to the Human Gene Mutation Database (HGMD), 13 out of the 17 mutations detected had been reported previously, and 4 were novel variants.
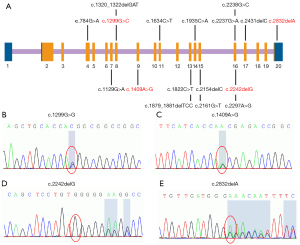
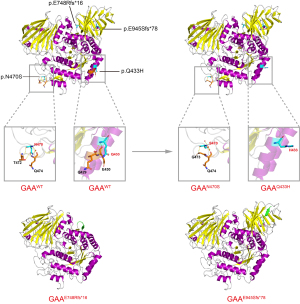
The four novel variants see Figure 2 located in exons of the GAA gene included two frameshift mutations and two missense mutations. Variants c.1299G>C (p.Q443H) and c.1409A>G (p.N470S) lead to changes in highly conserved amino acids (Figure 3). Five different algorithms (Mutation t@sting, Polyphen-2, SIFT, fathmm, and Mutation-Assessor) were used to predict the pathogenicity of the two novel missense variants (Table 3). PyMOL software was also applied to generate the structural figures of the four novel variants and their wide types (Figure 4). The homozygous mutation c.1409A>G (p.N470S) in patient 2 triggered LOPD with airway infections and respiratory failure onset; in patient 7, the c.1409A>G (p.N470S) and c.2161G>T (p.E721*) mutations were responsible for the pathogenicity of the disease. The two novel frameshift variants, c.2242delG (p.E748Rfs*16) and c.2832delA (p.E945Sfs*78), also caused early termination of protein synthesis and loss of protein function after the catastrophe point. Further, according to the guidelines of American College of Medical Genetics and Genomics (ACMG) (8), the novel missense mutation c.1299G>C (p.Q433H) can be rated as a likely pathogenic variant.
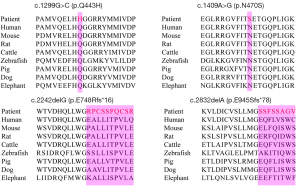
Table 3
| DNA change | Protein change | Mutation taster | Polyphen-2 | SIFT | Fathmm | Mutation-Assessor |
|---|---|---|---|---|---|---|
| c.1299G>C | p.Q433H | Polymorphism | Possibly damaging (score: 0.617) | Tolerated (score: 0.083) | Damaging (score: −3.2) | Medium (score: 2.42) |
| c.1409A>G | p.N470S | Disease causing | Probably damaging (score: 0.909) | Damaging (score: 0.034) | Damaging (score: −2.78) | Medium (score: 1.945) |
GAA, acid alpha-glucosidase; DNA, deoxyribonucleic acid; SIFT, sorting intolerant from tolerant.
Course and treatment
Patients 3 and 12 had scoliosis as the first symptom, for which they underwent posterior spinal correction surgery, and they were then diagnosed with LOPD through muscle biopsy, GAA activity testing, and GAA mutation analysis. Patients 2, 5, 6, 7, 8, 9, 11, and 13 required ventilator support due to laboured breathing.
In our study, only patients 4 and 7 received ERT. Patient 4 received 18 times the full dose (20 mg/kg), but did not have regular ERT treatments in the period from September 26, 2019 to December 10, 2020. Further, due to the high treatment cost and local healthcare policies, this patient has unfortunately not received ERT since December 2020. During treatment with ERT, the forced vital capacity of the patient remained unchanged. Recently, due to an increased heart rate while sleeping in a supine position, patient 4 has required intermittent non-invasive ventilation if he is positioned on his back. Patient 7 received several small doses of ERT and needed continuous non-invasive ventilation.
Discussion
Pompe disease represents a continuum of the disease spectrum. The categorization of its two subtypes is based on whether symptom onset occurs before or after 1 year of age. Typically, residual GAA activity causes LOPD to present in adulthood, presenting as proximal limb-girdle muscle weakness with prominent respiratory involvement (9). Furthermore, LOPD has broad phenotypic manifestations, including bulbar muscle involvement, scoliosis (10), osteoporosis (11), sleep apnoea and sleep-disordered breathing (12), gastrointestinal dysfunction (13), lower urinary tract and sphincter involvement, and fatigue, among others (14). The present study involved a cohort of 14 patients with LOPD from 13 families in mainland China. Proximal muscle weakness was the first prominent symptom in eight patients, while the other six patients presented with respiratory failure, chest congestion and asthma, and scoliosis, which made the LOPD difficult to diagnose. However, as the disease progressed, all patients exhibited different levels of limb-girdle muscle weakness and osteoporosis.
In our study, the median ages of the patients at symptom onset and diagnosis were 15.0 years (7–36 years) and 21.5 years (8–47 years), respectively. In comparison, in a Belgian cohort (15), the mean age at symptom onset was 28.9 years [standard deviation (SD): 15.8 years]; in a French cohort (16), the mean ages at disease onset and diagnosis were 34.6 years (SD: 20.1 years) and 43.1 years (SD: 17.5 years), respectively; and in an Austrian cohort (17), the mean symptom onset age was 24.9 years (SD: 13.9 years). Therefore, one of the key findings of the present study is that the patients were younger at disease onset and diagnosis than patients in studies from other countries, which suggests racial differences in LOPD pathogenicity. This finding is also consistent with the results a previous study of 59 patients with LOPD conducted in mainland China, which reported that the mean ages at disease onset and diagnosis were 14.9 (12.35) and 22.1 (10.08) years, respectively (18).
In our study, the median diagnostic delay (from initial symptom onset to diagnosis) was 3.0 years (0–22 years). However, the results reported for diagnostic delay vary considerably between countries. For instance, the diagnostic delay in cohorts from Austria, China, and Iran were 7.4, 5, and 3.3 years, respectively (17-19). Therefore, in the present study, the diagnostic delay was shorter than those previously observed. The shorter diagnostic gap is likely attributable to the use of fast dried blood spot analysis or other blood analysis of GAA activity, as well as the development of genetic testing technology. It also reflects the improving awareness of Pompe disease among patients and doctors.
Intravenous ERT with recombinant human GAA is the only drug to date to address the underlying cause of Pompe disease (20). It received FDA approval to treat both IOPD and LOPD in 2007, and was approved in mainland China in 2016. The clinical response to ERT varies between patients and is associated with various factors, such as age and the degree of muscle damage at the initiation of ERT, defective autophagy, and cross-reactive immunological material (CRIM) status. CRIM has been found to be an important predictor of clinical response in Pompe disease. CRIM-negative infantile patients usually present poorly, which is likely due to the development of a sustained high titer of neutralizing antibodies to recombinant human GAA (21). A limitation of ERT is that 20 mg/kg every week at the standard dose may be insufficient to prevent the long-term progress of the disease (22). Also, according to clinical assessments and follow-up results, a decline in pulmonary function was observed in our cohort, which is indicative of a deteriorating clinical course. However, the long-term clinical benefit of ERT remains to be demonstrated.
A total of 17 variants in the GAA gene were detected among the 14 patients in this study, of which 4 mutations were novel and 13 mutations had been reported previously. The two novel missense variants with damaging predicted results may be novel pathogenic sites of LOPD. Genetic analysis of the 14 patients revealed that the c.2238G>C (p.W746C) mutation was found at an allele frequency of 14.3% (4/28) and in 28.6% of patients (4/14), making it the most frequent mutation. This mutation was previously reported in 55.56% of patients with LOPD in a mainland Chinese study, with a high allele frequency of 27.08% (23). It was also reported in 58.1% (18/31) of Pompe patients in another cohort from mainland China (18). Therefore, its frequency was comparatively low in our cohort. The novel missense variant c.1409A>G (p.N470S) was one of the second most frequently detected mutations in our cohort, with a frequency of 10.7% (3/28). In our study, we indicated the pathogenicity of the two novel missense variants, c.1299G>C (p.Q433H) and c.1409A>G (p.N470S), using silico data and protein modelling, but cell and animal experiments were not performed to verify their pathogenicity. Therefore, these two novel missense variants should be classified as variants of uncertain significance. Furthermore, c.2832delA (p.E945Sfs*78) occurs in the last exon of GAA. According to the ACMG interpretation guidelines, care should be taken in applying PVS1 as a criterion to variants in the last exon of a gene, since it is unknown if the protein will merely be truncated and still functional without having undergone nonsense-mediated decay. There is not enough evidence to support c.2832delA (p.E945Sfs*78) as being pathogenic, so this variant must also be classified as a variant of uncertain significance. In future, we will carry out relevant basic research for further verification and investigation of these novel variants.
Interestingly, in patients 11 and 12 (who were brothers), who had the same mutations of the GAA gene, the symptom of disease onset was different. Through careful physical examination, the brothers were found to have declined proximal and axial muscle weakness, although patient 12 was still able to walk normally. The initial symptoms of LOPD vary depending on which muscles are more affected; furthermore, environmental factors, posture and gait, mode of life, and infection may also play roles in the course of disease onset and progression.
Limitations of our study are that the study population was small and follow-up visits varied greatly without uniform assessment. In future, the genotypes and phenotypes of LOPD need larger multicentre and longer follow-up studies.
Conclusions
This study has described the genetic features and clinical manifestations of 14 mainland Chinese patients with LOPD. The result that the c.2238G>C (p.W746C) mutation in the GAA gene was the most frequent mutation in our cohort is consistent with previous findings in southern Chinese patients with LOPD. This study has also identified four novel variants in patients with LOPD. These variants included c.1409A>G (p.N470S), which has not been reported before and was the second most frequent mutation among our patients; further research is required to verify the function of this mutation. Predicting the pathogenicity of these novel variants may increase the understanding of the genetic mutation spectrum of LOPD. The delay between symptom onset and diagnosis for Chinese patients with LOPD is similar to that in patients from other countries, thanks to improved detection techniques and awareness of Pompe disease in China. LOPD eventually results in wheelchair dependency and the need for a respirator, which emphasizes the necessity of prenatal diagnosis. Efforts should be made to improve awareness of the clinical and genetic characteristics of Chinese patients with LOPD among doctors and patients. However, the genotypes and phenotypes of LOPD require further research and exploration. Considering the high fatality rate of IOPD, the high disability rate of LOPD, and the high medical costs associated with the disease, prenatal diagnosis and neonatal enzymology screening are recommended.
Acknowledgments
We thank the study participants and their families for their involvement in this study, and for permitting their data to be used for scientific research.
Funding: This work was financially supported by the National Natural Science Foundation of China (No. 82071434), the Natural Science Foundation of Jiangsu (No. BK20201490), Nanjing Medical University Specific Disease Cohort Study Project (No. NMUC2019007A), Jiangsu Province “Six Talent Peak” High-Level Talent Selection and Training Programme (No. WSN-004), and the 511 Project.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3710
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3710
Peer Review File: Available at https://dx.doi.org/10.21037/atm-21-3710
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3710). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Review Board of The First Affiliated Hospital of Nanjing Medical University (2021-SR-415). The patients and their families gave informed consent for the use of their medical data for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen X, Liu T, Huang M, et al. Clinical and Molecular Characterization of Infantile-Onset Pompe Disease in Mainland Chinese Patients: Identification of Two Common Mutations. Genet Test Mol Biomarkers 2017;21:391-6. [Crossref] [PubMed]
- Lim JA, Li L, Raben N. Pompe disease: from pathophysiology to therapy and back again. Front Aging Neurosci 2014;6:177. [Crossref] [PubMed]
- Su X, Sheng H, Huang Y, et al. Clinical and GAA gene mutation analysis in 21 Chinese patients with classic infantile pompe disease. Eur J Med Genet 2020;63:103997. [Crossref] [PubMed]
- Tarnopolsky M, Katzberg H, Petrof BJ, et al. Pompe Disease: Diagnosis and Management. Evidence-Based Guidelines from a Canadian Expert Panel. Can J Neurol Sci 2016;43:472-85. [Crossref] [PubMed]
- Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, et al. Characterization of the human lysosomal alpha-glucosidase gene. Biochem J 1990;272:493-7. [Crossref] [PubMed]
- Buratti E, Peruzzo P, Braga L, et al. Deferoxamine mesylate improves splicing and GAA activity of the common c.-32-13T>G allele in late-onset PD patient fibroblasts. Mol Ther Methods Clin Dev 2021;20:227-36. [Crossref] [PubMed]
- Yang CC, Chien YH, Lee NC, et al. Rapid progressive course of later-onset Pompe disease in Chinese patients. Mol Genet Metab 2011;104:284-8. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 2008;372:1342-53. [Crossref] [PubMed]
- Haaker G, Forst J, Forst R, et al. Orthopedic management of patients with Pompe disease: a retrospective case series of 8 patients. ScientificWorldJournal 2014;2014:963861. [Crossref] [PubMed]
- Horvath JJ, Austin SL, Case LE, et al. Correlation between quantitative whole-body muscle magnetic resonance imaging and clinical muscle weakness in Pompe disease. Muscle Nerve 2015;51:722-30. [Crossref] [PubMed]
- Boentert M, Karabul N, Wenninger S, et al. Sleep-related symptoms and sleep-disordered breathing in adult Pompe disease. Eur J Neurol 2015;22:369-76, e27.
- Bernstein DL, Bialer MG, Mehta L, et al. Pompe disease: dramatic improvement in gastrointestinal function following enzyme replacement therapy. A report of three later-onset patients. Mol Genet Metab 2010;101:130-3. [Crossref] [PubMed]
- Chan J, Desai AK, Kazi ZB, et al. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol Genet Metab 2017;120:163-72. [Crossref] [PubMed]
- Vanherpe P, Fieuws S, D'Hondt A, et al. Late-onset Pompe disease (LOPD) in Belgium: clinical characteristics and outcome measures. Orphanet J Rare Dis 2020;15:83. [Crossref] [PubMed]
- Semplicini C, Letard P, De Antonio M, et al. Late-onset Pompe disease in France: molecular features and epidemiology from a nationwide study. J Inherit Metab Dis 2018;41:937-46. [Crossref] [PubMed]
- Löscher WN, Huemer M, Stulnig TM, et al. Pompe disease in Austria: clinical, genetic and epidemiological aspects. J Neurol 2018;265:159-64. [Crossref] [PubMed]
- Zhao Y, Wang Z, Lu J, et al. Characteristics of Pompe disease in China: a report from the Pompe registry. Orphanet J Rare Dis 2019;14:78. [Crossref] [PubMed]
- Nazari F, Sinaei F, Nilipour Y, et al. Late-onset Pompe disease in Iran, a clinical and genetic report. Muscle Nerve 2017;55:835-40. [Crossref] [PubMed]
- Hahn A, Schänzer A. Long-term outcome and unmet needs in infantile-onset Pompe disease. Ann Transl Med 2019;7:283. [Crossref] [PubMed]
- Bali DS, Goldstein JL, Banugaria S, et al. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am J Med Genet C Semin Med Genet 2012;160C:40-9. [Crossref] [PubMed]
- Khan AA, Case LE, Herbert M, et al. Higher dosing of alglucosidase alfa improves outcomes in children with Pompe disease: a clinical study and review of the literature. Genet Med 2020;22:898-907. [Crossref] [PubMed]
- Liu X, Wang Z, Jin W, et al. Clinical and GAA gene mutation analysis in mainland Chinese patients with late-onset Pompe disease: identifying c.2238G > C as the most common mutation. BMC Med Genet 2014;15:141. [Crossref] [PubMed]
(English Language Editors: Chelsea Mullens and J. Reynolds)


