
This article has an erratum available at: http://dx.doi.org/10.21037/atm-2022-8 the article has been update on 2022-04-01 at here.
Collagen triple helix repeat containing-1 exerts antifibrotic effects on human skin fibroblast and bleomycin-induced dermal fibrosis models
Introduction
Systemic scleroderma (SSc) is an autoimmune disease resulting from genetic predisposition or environmental stimuli. It is characterized by severe microvasculature changes, dramatic inflammatory and immunological alterations, and fibrosis of the skin and internal organs. However, the mechanism of interaction between these 3 major abnormalities has yet to be described (1,2).
Collagen triple helix repeat containing-1 (CTHRC1) is expressed mostly in adventitial fibroblasts and neointimal smooth muscle cells in balloon-injured vessels. It can promote cell migration by decreasing the deposition of collagen matrix and plays a key role in tissue repair following injury (3). A number of studies have revealed that CTHRC1 upregulation is predictive of a poor prognosis in patients with various tumors. The overexpression of Cthrc1 enhances the migration and invasion of tumor cells, indicating that it is a feasible independent prognostic factor for patients with cancer. LeClair et al. observed that adventitial collagen deposition and neointimal lesion formation were reduced in transgenic mice overexpressing CTHRC1 following carotid artery ligation (4). All of the above studies suggest that CTHRC1 could have a major clinical application in reducing collagen deposition.
Transforming growth factor-β (TGF-β) is understood to be the leading profibrotic factor in sclerotic disorders (5). CTHRC1 has been identified as a cell type-specific inhibitor of TGF-β, which, in sequence, impacts collagen deposition, neointimal formation, and smooth muscle cell differentiation (4,6,7). Our previous work investigated the inhibitory effects of CTHRC1 on TGF-β1-stimulated collagen deposition in keloid fibroblasts (8). However, until now, the expression and regulatory mechanisms of CTHRC1 in SSc have not been explored.
In this study, we firstly measured the expression of CTHRC1 and TGF-β1 in scleroderma fibroblasts, and then examined the expression and synthesis of collagen type I in normal human dermal fibroblasts stimulated with TGF-β1. Finally, we constructed a bleomycin (BLM)-induced dermal fibrosis mouse model to investigate the protective effect of CTHRC1 against dermal fibrosis in mice.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-21-1884).
Methods
Cell culture
A normal human dermal fibroblast cell line was bought from Kunming Cell Bank of the Chinese Academy of Sciences. Fibroblasts were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and grown at 37 °C in a 5% CO2 atmosphere. The growth medium was changed every 3 to 4 days.
Cell proliferation assay
Cells were seeded on 96-well plates at a density of 5×103/well. Using the 3-(4,5-dimethyl-2-thiazolyl)-2,5 -diphenyl-2H-tetrazolium bromide (MTT) assay, The proliferative activity of fibroblasts treated with TGF-β1 or recombinant CTHRC1 (rCTHRC1) was assessed. The absorbance was detected at a reference wavelength of 570 nm. Recombinant CTHRC1 was kindly supplied by Prof. Chen (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China).
Real-time reverse transcriptase-polymerase chain reaction
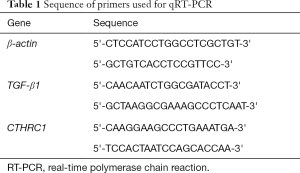
Total RNA was extracted using TRIzol reagent (Life Technologies, USA). The RNA concentration and quality were tested using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA). Complementary DNA (cDNA) was synthesized through reverse transcription of 1 µg of RNA using the RevertAid First Strand Synthesis kit (Fermentas, Glen Burnie, MD, USA). Then, quantitative real-time polymerase chain reaction (qRT-PCR) analysis was carried out using SYBR® Premix Ex Taq (Takara Bio Inc., Shiga, Japan) following the manufacturer’s instructions. Each sample was analyzed in triplicate, with β-actin serving as an internal control. Sequence of primers used are list in Table 1.
Full table
Western blot
Proteins were extracted from human tissues and cells using RIPA lysis buffer, and a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA) was used to measure the protein concentration. Following separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), equal amounts of protein were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were incubated with collagen type I monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) after processing in the blocking buffer. Protein expression was normalized to β-actin expression (Santa Cruz Biotechnology, Santa Cruz, CA, USA). An imaging densitometer was employed for quantitative analysis of the blotting images.
Collagen synthesis assay
Collagen synthesis was examined using a 3H-proline incorporation assay as previously described (9), with some optimizations. Cells were counted with a liquid scintillation counter, which indicated the amount of newly synthesized collagen. Collagen synthesis was normalized to the number of cells.
Model establishment and treatment
Six-week-old specific pathogen-free female BALB/c mice (weight: 18 to 22 g) were purchased from the animal research center at Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China. All animal experimental steps were carried out in adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and with the approval of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China (No.: Sichuan Provincial People’s Hospital-D-2013-99). The BLM-induced dermal fibrosis mouse model was established following previously described methods (10). Briefly, BLM (Nippon Kayaku Co., Ltd., Tokyo, Japan) was dissolved in 1 mg·mL−1 phosphate-buffered saline (PBS). In the BLM mice, after sterilization by filtration, 100 µL BLM solution was subcutaneously injected into the shaved back of mice using a 27-gauge needle, the injections were repeated daily for a total of 21 days. The control mice were injected with an equal volume of PBS. After the successful establishment of the model, the BLM mice were randomly divided into the PBS group (8 mice), the rCTHRC1 group (8 mice), the TGF-β1 group (8 mice), and the rCTHRC1 + TGF-β1 group (8 mice). The mice were intraperitoneally injected with the designated drugs every 2 days for a 20-day period. The general conditions of the mice including their activity and weight were observed daily.
Histological examination
Dermal tissues from the experimental animals were sliced into 5-µm sections. For structured observation or detection of collagen deposits, the sections were stained with hematoxylin and eosin or Masson’s trichrome stain respectively, as instructed by the manufacturers.
Immunohistochemistry
Tissue samples were volunteer contributed by patients with SSc who had received treatment in the Department of Dermatology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China (No.: Sichuan Provincial People’s Hospital-D-2013-350) and informed consent was taken from all the patients. Immunohistochemical staining was carried out to detect the expression of CTHRC1, TGF-β1, and type I collagen in the dermal tissues using primary antibodies of CTHRC1, TGF-β1, and type I collagen (all from Abcam, Cambridge, MA), respectively. Immune-specificity controls were included in all experiments.
Statistical analysis
SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for all data analyses. Data were presented as mean ± standard error of mean (SEM). A value of P<0.05 was considered statistically significant. Differences were tested for significance using the independent t test or Mann-Whitney U test. All experiments were carried out in triplicate and repeated at least 3 times.
Results
CTHRC1 is upregulated in SSc dermal tissues
To clarify the biological function of CTHRC1 in SSc fibroblasts, we firstly investigated the expression of CTHRC1 in SSc dermal tissue samples compared to normal dermal tissues. Immunohistochemical staining revealed CTHRC1 to be dramatically upregulated in SSc dermal fibroblasts. Furthermore, elevated expression of TGF-β1 was detected in SSc dermal fibroblasts compared to the normal controls (Figure 1), which was consistent with the observations of previous studies.

CTHRC1 attenuates TGF-β1-induced proliferation, collagen expression, and synthesis of fibroblasts
Although the activation mechanism of SSc fibroblasts is unknown, some traits of SSc fibroblasts match those of healthy fibroblasts stimulated with TGF-β1 (11,12). First, we assessed human dermal fibroblast activity after 24 hours of stimulation with 10 ng·mL−1 TGF-β1. The results revealed that TGF-β1 treatment led to a dramatic increase in fibroblast proliferation. Next, we treated TGF-β1-stimulated human dermal fibroblasts with rCTHRC1 and further observed the effects of CTHRC1 on fibroblasts. We found that CTHRC1 significantly inhibited the proliferation of fibroblasts (Figure 2A).

To further probe the inhibitory effects of CTHRC1, we measured the expression of collagen type I. The expression of collagen type I messenger RNA in fibroblasts was significantly increased by TGF-β1, and this effect was reversed by rCTHRC1 at 0.1 mg·mL−1 (Figure 2B). In line with the reduction of collagen expression, collagen synthesis in TGF-β1-stimulated human dermal fibroblasts was inhibited by rCTHRC1, as evidenced by the measurement of 3H-proline incorporation (Figure 2C). Furthermore, rCTHRC1 had no effect on cell viability.
The role of CTHRC1 in BLM-induced skin fibrosis
To unearth the function of CTHRC1 in dermal fibrosis in vivo, a BLM-induced dermal fibrosis mouse model was successfully established. First, Masson’s trichrome staining was carried out to evaluate collagen deposition, and the type I collagen expression was assessed by immunohistochemical staining. The results showed that BLM significantly increased collagen deposition in dermal tissues and significantly upregulated type I collagen expression compared with the controls. The expression of CTHRC1 was also examined by immunohistochemistry, which showed obvious upregulation of CTHRC1 in fibrotic tissues from BLM-treated mice. Significant upregulation of TGF-β1 was also observed in fibrotic dermal tissues (Figure 3).

CTHRC1 reduces collagen deposition in mice with BLM-induced dermal fibrosis
Following the research findings described above, we next explored the antifibrotic effects of CTHRC1 in a mouse dermal fibrosis model induced by BLM. We observed that BLM significantly increased collagen deposition and increased both the protein level of type I collagen and the hydroxyproline content in the skin tissues, while CTHRC1 treatment markedly reduced these changes. As shown in Figure 4, rCTHRC1 dramatically reduced the protein level of type I collagen and the hydroxyproline content. Thus, there is consistency between our in vitro and in vivo data from normal human skin fibroblast experiments and BLM-induced dermal fibrosis mouse experiments, respectively, suggesting that CTHRC1 may hold clinical promise for the treatment of fibrotic disorders such as SSc.

Discussion
CTHRC1 is recognized as a positive regulator involved in various physiological and pathological processes, including antifibrotic activity and tissue repair remodeling (3,8,13-15). Moreover, it could have extensive clinical applications, due to its ability to inhibit collagen deposition. An earlier study showed that CTHRC1 upregulation could prevent the progression of cholestatic liver fibrosis and could even cure hepatic fibrosis which had commenced (16).
To a great degree, vascular remodeling after injury is controlled by TGF-β, and the feasible mechanism of collagen regulation by CTHRC1 is considered to be mutually connected to CTHRC1 and the TGF-β signaling pathway. CTHRC1 is stimulated by TGF-β1 binding to the promoter of hepatic stellate cells via phospho-Smad3, and the signal descends to accelerate the phospho-Smad3 degradation. Thus, CTHRC1 and phospho-Smad3 are deemed to be negatively correlated in the TGF-β pathway (17).
Our previous work demonstrated that overproduction of CTHRC1 inhibited the TGF-β1-induced effects on keloid fibroblasts (8). In the present study, we firstly showed that CTHRC1 could inhibit human dermal fibroblast collagen deposition induced by TGF-β1. Further investigation showed that CTHRC1 alleviated BLM-induced dermal fibrotic changes in model mice.
The potent profibrogenic cytokine TGF-β is widely known to play a crucial role in hepatic fibrosis processes. In hepatic fibrosis animal models, encouraging results have been gained through blocking the TGF-β pathway (18,19); however, human data have proved disappointing due to TGF-β playing a dominant role in many aspects of cell biological functions (18). Several studies have suggested that a therapeutic dose of a neutralizing monoclonal antibody against TGF-β1 alleviated and even reversed fibrosis in several animal models (20-22). The efficacy of a systematically administrated anti-TGF-β1 drug was non-existent in the treatment of patients with SSc accompanied with severe side effects (23). In short, CTHRC1 is a promising therapeutic target for dermal fibrosis.
To our knowledge, this is the first study to demonstrate that CTHRC1 exerts antifibrotic effects on dermal fibrosis. We have provided evidence that CTHRC1 may be a potential drug for dermal fibrosis therapy, although further studies are needed.
Conclusions
In the present study, we have evidenced the antifibrotic effects of CTHRC1 in TGF-β1-stimulated human skin fibroblasts and BLM-induced dermal fibrosis in mice. As far as we know, it is the first report to present CTHRC1 as a potential therapeutic option for dermal fibrosis.
Acknowledgments
Funding: This work was funded by grants from the National Natural Science Foundation of China (grant No. 81301372).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-1884
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-1884
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-1884). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China (No.: Sichuan Provincial People’s Hospital-D-2013-350) and informed consent was taken from all the patients. All animal experimental steps were carried out in adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and with the approval of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China (No.: Sichuan Provincial People’s Hospital-D-2013-99).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levis B, Burri A, Hudson M, et al. Sexual activity and impairment in women with systemic sclerosis compared to women from a general population sample. PLoS One 2012;7:e52129 [Crossref] [PubMed]
- Akamata K, Asano Y, Aozasa N, et al. Bosentan reverses the pro-fibrotic phenotype of systemic sclerosis dermal fibroblasts via increasing DNA binding ability of transcription factor Fli1. Arthritis Res Ther 2014;16:R86. [Crossref] [PubMed]
- Pyagay P, Heroult M, Wang Q, et al. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res 2005;96:261-8. [Crossref] [PubMed]
- LeClair RJ, Durmus T, Wang Q, et al. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res 2007;100:826-33. [Crossref] [PubMed]
- Denton CP, Abraham DJ. Transgenic analysis of scleroderma: understanding key pathogenic events in vivo. Autoimmun Rev 2004;3:285-93. [Crossref] [PubMed]
- Durmus T, LeClair RJ, Park KS, et al. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr Patterns 2006;6:935-40. [Crossref] [PubMed]
- LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med 2007;17:202-5. [Crossref] [PubMed]
- Li J, Cao J, Li M, et al. Collagen triple helix repeat containing-1 inhibits transforming growth factor-b1-induced collagen type I expression in keloid. Br J Dermatol 2011;164:1030-6. [Crossref] [PubMed]
- Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension 1998;31:943-8. [Crossref] [PubMed]
- Yamamoto T, Takagawa S, Katayama I, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol 1999;112:456-62. [Crossref] [PubMed]
- Leroy EC, Smith EA, Kahaleh MB, et al. A strategy for determining the pathogenesis of systemic sclerosis. Is transforming growth factor beta the answer? Arthritis Rheum 1989;32:817-25. [PubMed]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol 1990;6:597-641. [Crossref] [PubMed]
- Kimura H, Kwan KM, Zhang Z, et al. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One 2008;3:e3174 [Crossref] [PubMed]
- Takeshita S, Fumoto T, Matsuoka K, et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 2013;123:3914-24. [Crossref] [PubMed]
- Tang L, Dai DL, Su M, et al. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res 2006;12:3716-22. [Crossref] [PubMed]
- Bian Z, Miao Q, Zhong W, et al. Treatment of cholestatic fibrosis by altering gene expression of Cthrc1: Implications for autoimmune and non-autoimmune liver disease. J Autoimmun 2015;63:76-87. [Crossref] [PubMed]
- Deng YR, Ma HD, Tsuneyama K, et al. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun 2013;46:25-34. [Crossref] [PubMed]
- Mehal WZ, Iredale J, Friedman SL. Scraping fibrosis: expressway to the core of fibrosis. Nat Med 2011;17:552-3. [Crossref] [PubMed]
- Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int 2006;26:8-22. [Crossref] [PubMed]
- Ikawa Y, Ng PS, Endo K, et al. Neutralizing monoclonal antibody to human connective tissue growth factor ameliorates transforming growth factor-beta-induced mouse fibrosis. J Cell Physiol 2008;216:680-7. [Crossref] [PubMed]
- Ling H, Roux E, Hempel D, et al. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013;8:e54499 [Crossref] [PubMed]
- McCormick LL, Zhang Y, Tootell E, et al. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol 1999;163:5693-9. [PubMed]
- Denton CP, Merkel PA, Furst DE, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 2007;56:323-33. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


