
Glucagon-like peptide-1 (GLP-1) improved diabetic lung fibrosis via AMPK and microRNA-27a (miR-27a)
Introduction
Vascular damage is a fundamental physiological process that underpins chronic complications seen in diabetes mellitus. As the lungs have the largest capillary network in the human body, diabetes can accelerate lung function decline (1). In Type 2 diabetes mellitus, the decline of pulmonary function was 2–3 times faster than that in healthy adults, and the risk of pulmonary fibrosis was significantly higher (OR =4.06, 95% CI: 1.80–9.15) (2).
Proliferation of extracellular matrix is the key mechanism for lung tissue damage in diabetes mellitus. Glucagon-like peptide-1 (GLP-1) analogues have been widely used in the treatment of diabetes mellitus. There is increasing evidence that GLP-1 has an extrapancreatic effect on organ fibrosis. After 12 weeks of intervention with the GLP-1 analogue liraglutide in diabetic rats, the expression of collagen type (Col-) III in lung tissue of Type 2 diabetic rats was significantly decreased (3). In the high-glucose condition, the expression of fibronectin and Col-III was decreased significantly after the intervention of GLP-1 in MRC-5 cells (4). These results suggest that GLP-1 significantly improves proliferation of extracellular matrix in the diabetic lung, and that this improvement is unrelated to blood sugar control (5).
The microRNA-27a (miR-27a) family is highly expressed in lung and heart tissues. Kang et al. (6) found that after 3 weeks of hypoxic exposure (10%), the lung tissue of C57BL/6 mice showed significantly increased expression of miR-27a, while the expression of peroxisome proliferator-activated receptor γ (PPARγ) decreased. Over-expression of miR-27a could inhibit the expression of PPARγ (7). PPARγ is expressed in various kinds lung cells, including alveolar macrophages, epithelial cells, fibroblasts, and vascular endothelial cells. PPARγ agonists can reduce the transformation of fibroblasts to myofibroblasts, and promote the transformation of fibroblasts into adipocytes, thereby inhibiting proliferation.
Our previous results showed that AMPK activation significantly inhibited expression of miR-27a (8). Miao et al. found that GLP-1 analogues can promote the proliferation of insulin β cells through the AMPK/mTOR signaling pathway (9). Ben-Shlomo et al. also found that GLP-1 can inhibit the formation of fatty liver through the AMPK pathway (10).
This study aims to further explain the correlations between miR-27a, AMPK, and MRC-5 cell activity. With MRC-5 cells as the research subject, the influence of GLP-1 on the proliferation of the pulmonary extracellular matrix through AMPK/miR-27a regulation is investigated.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-21-869).
Methods
Cell culture
Human embryonic lung fibroblast (MRC-5) cells—purchased from Cell Culture Center of Chinese Academy of Medical Sciences (Beijing, China)—were cultured in low glucose Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum, 1% L-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin, and kept in humidified atmosphere containing 5% CO2 at 37 °C.
Cell transfection
The miR-27a gain-of-function study was performed using miR-27a mimics, inhibitor (50 nM, GenePharma, Shanghai, China) and their negative control (50 nM), respectively, on the MRC-5 fibroblast. The miR-27a mimics sequence is 5'-UUCACAGUGGCUAAGUUCCGC-3'. The miR-27a inhibitor sequence is 5'-GCGGAACUUAGCCACUGUGAA-3'. MRC-5 cells were transfected using Lipofectamine™ RNAiMax (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The relative level of miR-27a in transfected MRC-5 cells was detected by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
qRT-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, CA, USA) or miRNeasy Mini Kit (QIAGEN) according to manufacturer’s instructions. RNA quality and quantity were measured using a nanodrop spectrophotometer (ND-1000, Nanodrop Technologies, MA, USA). RNA integrity was determined by gel electrophoresis. The samples were reverse transcribed to cDNA using EzOmicsTMSYBR qPCR Kit (Biomics, BK2200, Nantong, China). The expression of miRNA was quantified with qRT-PCR using SYBR® Premix Ex Taq™ (Takara, RRoc41A, Kusatsu, Japan). The relative expression of miR-27a was calculated using the expression of U6 small nuclear RNA as the reference. The sequence-specific forward primers for mature miR-27a and U6 internal control were as follows: for miR-27a, 5'-CGCATTCACAGTGGCTAAG-3'; and for U6, 5'-TGCGGGTGCTCGCTTCGGC-3'.
Collection of cell lysate
MRC-5 cells were treated with liraglutide, compound C, or miR-27a inhibitor. Cultured MRC-5 cells were then harvested and extracted in RIPA lysate buffer.
Western blotting
Protein samples were separated by SDS-PAGE gels and transferred to nitrocellulose membrane. The membranes were washed with Tris-buffered saline supplemented with 0.05% NP-40 and 5% non-fat dry milk for 30 minutes at 37 °C. Immunoblotting was performed following standard procedures, and the signals were detected using chemiluminescence reagents (Syngene). Gene Tool analyzes the protein expression of the fibrosis-related genes. Primary antibodies were directed against: PPARγ (1:1,000, Proteintech, 16643-1-AP), Col-IV (1:1,000, Proteintech, 55131-1-AP), Fibronectin (1:1,000, Proteintech, 15613-1-AP), NF-κB p65 (1:2,000, Proteintech, 10745-1-AP), α-SMA (1:4,000, Proteintech, 14395-1-AP), TGF-β1 (1:2,000, Proteintech, 21898-1-AP), caspase-3 (1:1,000, CST, #5140) and β-actin (1:4,000, Proteintech, 20536-1-AP).
Cell counting kit-8 (CCK-8) detected the proliferation of MRC-5 cells
Cell proliferative rates of MRC-5 cells lines were evaluated with the CCK-8 assay (MedChemExpress, China) according to the manufacturer’s instructions. The above glioma cells were treated with 10, 100, 1,000 nM liraglutide respectively, and plated into 96-well plates (2×103 cells per well) containing media. The MRC-5 cells were then cultured for 24 hours and incubated with CCK-8 reagent at a final concentration of 10 μL/mL for 4 hours at 37 °C. Before reading the plate, it was mixed gently on an orbital shaker for 1 minute to ensure homogeneous distribution of color. The absorbance was then measured at 450 nM with a Thermomax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Each experiment was repeated three times.
Statistical analysis
Results were presented as a mean±standard deviation. Statistical analyses were assessed using a Student’s t-test between two groups and one-way ANOVA with multiple groups. All analyses were performed with SPSS 19.0 (SPSS Inc., USA) and a value of P<0.05 was considered statistically significant.
Results
Hyperglycemia promoted the expression of miR-27a and decreased the expression of fibrosis-related genes in human embryonic lung fibroblast (MRC-5) cells
In this study, MRC-5 cells were selected as the research subject. MRC-5 cells were cultured in three groups: normal control group (NC, DMEM medium containing 5 mM of glucose), hyperosmotic group (M, DMEM medium containing mannitol equal to 25 mM glucose group’s osmotic pressure), and hyperglycemic group (HG, DMEM medium containing 25 mM of glucose). qRT-PCR was used to detect the expression of miR-27a in the three groups of cells after 48 hours of culture (Figure 1A). Compared with normal control group, there was no difference in the expression of miR-27a in hyperosmotic group, while the expression of miR-27a in hyperglycemic group was significantly upregulated (P<0.01).
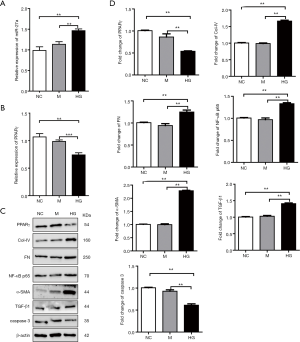
PPARγ plays an important role in the pathogenesis of organ fibrosis. We detected the mRNA expression of PPARγ in all three groups of cells (Figure 1B). Compared with the normal control group, the expression of PPARγ in the hyperosmotic group was not different, while the expression of PPARγ in the hyperglycemic group was significantly down-regulated (P<0.01).
Extracellular matrix proliferation is a key problem in diabetic lung damage. We further examined the expression of fibrosis-related genes. The expression of PPARγ protein decreased significantly in cells cultured with high-glucose medium. Accordingly, compared with control group, the expression of extracellular matrix-related genes Col-IV and fibronectin, the expression of extracellular matrix synthesis pathway-related genes NF-κB p65, and the expression of fibroblast differentiation and proliferation-related genes α-SMA and TGF-β1 increased significantly. The expression of caspase-3 protein decreased significantly (P<0.01, Figure 1C,D).
The effects of miR-27a over-expression or inhibited expression on fibrosis-related genes expression in MRC-5 cells
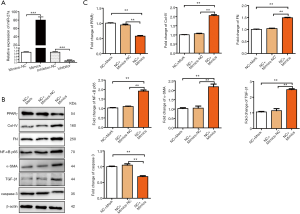
In the hyperglycemic condition, the expression of miR-27a and PPARγ in MRC-5 cells changed negatively. Kang et al. (6) found that PPARγ 3'-UTR had a binding site of miR-27a in pulmonary vascular cells, which confirmed that PPARγ was the target gene of miR-27a. We speculate that miR-27a directly regulated pulmonary extracellular matrix proliferation by its target PPARγ. MRC-5 cells were transfected with miR-27a mimics, and the expression of miR-27a and PPARγ were detected by qRT-PCR. miR-27a mimics significantly increased the level of miR-27a and inhibited the expression of PPARγ (Figure 2A,B). Compared with the normal control group, the expression of Col-IV, fibronectin, NF-κB p65, α-SMA and TGF-β1 increased significantly. The expression of caspase-3 protein decreased significantly (P<0.01, Figure 2C,D).
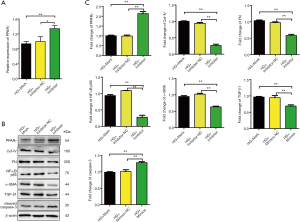
We further examined the effects of miR-27a inhibitor on these genes’ expression in MRC-5 cells. After 48 hours of transfection, MRC-5 cells were cultured in high-glucose medium and transfected by miR-27a inhibitor for 24 hours. PPARγ protein expression in high-glucose plus inhibitor group was significantly up-regulated (P<0.01) compared with normal control group (Figure 3A). Further collecting proteins from the three groups of cells showed that in the high-glucose condition, down-expression of miR-27a in MRC-5 cells significantly inhibited the protein expression of Col-IV and fibronectin, NF-κB p65, α-SMA and TGF-β1, and increased the expression of caspase-3 and MMP-2 (Figure 3B,C). The results suggested that the expression of genes related to fibrosis in MRC-5 cells can be altered by altering the level of miR-27a.
GLP-1 promoted the expression of miR-27a and the AMPK signaling pathway was involved in the biological process of GLP-1 regulating miR-27a expression in MRC-5 cells
Our previous studies shown that GLP-1 improved the proliferation of extracellular matrix in diabetic lung significantly; however, the mechanism remains unclear. We first examined the effects of different concentrations of GLP-1 on the proliferation of MRC-5 cells. There were no differences in cell proliferation between 10, 100, 1,000 nM treatment groups and the control group (Figure 4A).
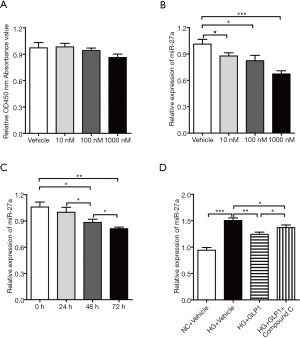
We further examined the expression of miR-27a in MRC-5 cells treated with different concentrations of GLP-1. MRC-5 cells were treated with 10, 100 and 1,000 nM GLP-1 for 48 hours respectively. The expression of miR-27a was down-regulated in 10 and 100 nM groups compared with the control group (P<0.05), and significantly down-regulated in the 1,000 nM GLP-1 intervention group (P<0.01). It is suggested that the expression of miR-27a was related to the concentration of GLP-1 (Figure 4B).
MRC-5 cells were cultured in DMEM with 25 mM glucose and were treated with 10 nM GLP-1 for 24, 48 or 72 hours respectively. There was no significant difference in the expression of miR-27a between the 24-hour treatment group and the control group. After 48 hours of treatment, the expression of miR-27a decreased (P<0.05), and the expression of miR-27a decreased further (P<0.01) in the 72-hour treatment group (Figure 4C). Statistical analysis shows that the change of miR-27a expression was correlated with the treatment time of GLP-1.
A large number of studies suggested that GLP-1 played its biological role through the AMPK signaling pathway. Hence, we endeavored to determine whether the AMPK signaling pathway is also involved in GLP-1’s regulation of miR-27a expression in MRC-5 cells. We pretreated the MRC-5 cell with GLP-1 analogue; this showed that the expression of miR-27a in GLP-1 group was significantly lower than that in Vehicle group. We then treated MRC-5 cells with compound C (AMPK inhibitor) before supplying them with GLP-1. The expression of miR-27a increased significantly after inhibition of AMPK signal, while it was still lower than that in Vehicle group. It was indicated that inhibition of AMPK signal could not completely relieve the inhibitory effect of GLP-1 on miR-27a expression (Figure 4D). These results suggested that AMPK signaling pathway was indeed involved in the biological process of GLP-1’s regulation of miR-27a expression in MRC-5 cells; however, GLP-1 may also regulate the expression of miR-27a through other additional signaling pathways.
In conclusion, the results suggest that GLP-1 may up-regulate the expression of PPARγ by inhibiting the expression of miR-27a, thus improving the accumulation of extracellular matrix in the diabetic lung.
Discussion
Chronic complications of diabetes mellitus may cause of death and disability. The lung is a target organ of pathology in diabetes mellitus and epidemiological studies have shown that diabetic patients have certain restrictive ventilation dysfunction, diffusion dysfunction and small airway dysfunction. Compared with non-diabetic patients, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and diffusing capacity of carbon monoxide (DLCO) is reduced by 10–30% in diabetic patients with no smoking history and lung diseases. Therefore, diabetes in patients with acute or chronic heart or lung diseases may lead to severe respiratory failure (11). Exploring the prevention and treatment strategies for lung damage in diabetic patients will help to improve the clinical prognosis in this population.
Mexican case-control study showed that T2DM is the most important independent risk factor associated with pulmonary fibrosis (12). It has been shown that alveolar epithelial cells and the endothelial capillary basal lamina are significantly thicker in diabetic patients than in control subjects (13). Diabetes mellitus significantly increases mortality in Idiopathic pulmonary fibrosis patients (14). Advanced glycation end products accumulation in the lung due to hyperglycemia may increase lung oxidative stress, resulting in lung fibrotic changes (15). Hyperglycemia induces oxidative stress, reactive oxygen species and reactive nitrogen species generation, and impairment of the capacity of the antioxidative defense system are the major causes of type 1 diabetes mellitus-induced lung fibrosis (16). Hyperinsulinemia, a major feature of type 2 diabetes mellitus, increases collagen deposition in the lungs and stimulates airway hyperresponsiveness through increasing contractile effects on airway smooth muscle cells (17).
Pathological extracellular matrix proliferation is a key change seen in lung damage in diabetes mellitus patients. In our previous research, we found that the alveolar capillary basement membrane was diffusely thickened in lungs of Type 2 diabetes mellitus patients and rabbits (18,19). PPARγ plays an important role in the mechanism of organ fibrosis. PPARγ also directly inhibits the expression of NF-κB, regulates the expression of matrix metalloproteinases (MMP-9) and MMP-2 at the gene level by interacting with NF-κB, and reduces the expression of connective tissue growth factor (CTGF) (7). PPARγ agonists also significantly inhibit the expression of TGF-β1/Smad and TGF-β1/JNK signal transduction pathways induced by extracellular matrix proliferation (20,21). The number of fibroblasts and the expression of α-SMA, fibronectin, Col-I, and Col-III in lung tissue of spontaneous type 2 diabetic OLETF rats have been shown to increase significantly (22). Compared with the normal control group, the expression of PPARγ protein in MCF-5 cells in the high-glucose group was significantly down-regulated (P<0.01, see Figures 3 and 4). Furthermore, the expression of extracellular matrix-related genes Col-IV and fibronectin, the expression of extracellular matrix synthesis pathway related genes NF-κB p65, and the expression of α-SMA and TGF- β1 related to differentiation and proliferation of fibroblasts were significantly increased. The expression of apoptosis-related gene caspase-3 decreased significantly.
miRNAs inhibited the expression of the target gene by specifically recognizing the corresponding target site on the target gene 3'UTR and binding to it by base complement pairing. Kang et al. found that the expression of miR-27a in lung tissue of C57BL/6 mice increased after exposure to hypoxia (10%) for 3 weeks, while the expression of PPARγ decreased significantly. Over-expression of miR-27a inhibited the expression of PPARγ, and luciferase reporter gene test confirmed that PPARγ was the target gene of miR-27a (Figure 1). It is speculated that miR-27a may play an important role in the proliferation of extracellular matrix in diabetic lung. Compared with the normal control group, the expression of miR-27a in the high-glucose group was significantly upregulated (P<0.01). Under the high-glucose condition, the expression of miR-27a and PPARγ in MRC-5 cells showed a reverse relationship, and the expression of Col-IV, fibronectin, NF-κB p65, α-SMA, and TGF-β1 were also changed accordingly. We speculated that miR-27a regulated the proliferation of extracellular matrix in the lung by targeting PPARγ. We then used miR-27a mimics and inhibitor transfected MRC-5 cells cultures with high-glucose medium. Over-expression of miR-27a inhibited the expression of extracellular matrix-related genes Col-IV and fibronectin protein, decreased the expression of extracellular matrix synthesis pathway related gene NF-κB p65 protein, down-regulated the expression of α-SMA and TGF-β1 proteins, and promoted the expression of apoptosis-related genes caspase-3 and MMP-2 protein (Figure 2). These results suggested that the expression of extracellular matrix proliferation-related genes can be changed by regulating the expression of miR-27a in MRC-5 cells at high glucose levels.
GLP-1 analogues have been widely used in hypoglycemic therapy for diabetes mellitus. Tang et al. (23) observed that the thickness of the alveolar capillary basement membrane in OLETF rats decreased significantly after GLP-1 intervention. After GLP-1 intervention in MRC-5 cultured in high-glucose medium, the expression of fibronectin, Col-III in MRC-5 cells decreased significantly. The results suggested that GLP-1 significantly improved the proliferation of extracellular matrix in diabetic lung; however, the exact mechanism for this was unclear.
After 8 weeks of intervention with GLP-1 analogues in db/db mice, it was found that the thickening of renal capillary basement membrane, renal TGF- β1 and Col-IV expression in db/db mice were significantly reduced, but this improvement was not related to blood glucose control. It is suggested that GLP-1 may improve the proliferation of extracellular matrix in the diabetic lung through a glucose-independent way. MRC-5 cells were treated with 10 nM GLP-1 for 24, 48 or 72 hours, respectively. Compared with the control group, there was no difference in the expression of miR-27a in the 24-hour treatment group. The expression of miR-27a in MRC-5 cells was significantly down-regulated in the 48- and 72-hour treatment groups (P<0.01). MRC-5 cells were then treated with 10, 100 or 1,000 nM GLP-1 for 48 hours. The expression of miR-27a in all of the three groups was significantly down-regulated (P<0.01). It is suggested that the effect of GLP-1 on the expression of miR-27a is time- and concentration-dependent. Thus, GLP-1 may upregulate the expression of PPARγ via down-regulated miR-27a, leading to improved proliferation of extracellular matrix in the diabetic lung. Furthermore, we used CCK-8 to detect the effect of GLP-1 on the proliferation of MRC-5 cells. There was no significant difference in cell proliferation level between the four groups.
The function of GLP-1 is mediated by the GLP-1 receptor. GLP-1 activates the Gα subunit by binding to its receptor, thus activating adenylate cyclase, increasing intracellular cyclic adenosine monophosphate (cAMP) level, and activating protein kinase A (PKA). Foreign studies have found that the GLP-1 analogue liraglutide activated AMPK in mouse muscle cells and regulated the translocation of the glucose cotransporter 4 (GLUT4) in mouse muscle cells (24). Ben-Shlomo et al. also found that GLP-1 inhibited the formation of fatty liver through the AMPK pathway (10). It was shown that activation of AMPK significantly inhibited the expression of the miR-27a family in primary cultured hepatocytes in C57BL/6 mice (25). So we expected to determine whether the AMPK signaling pathway was also involved in the biological process of GLP-1 regulating miR-27a expression in MRC-5 cells.
MRC-5 cells were treated with GLP-1 analogues. Compared with the control group, the expression of miR-27a in the GLP-1 intervention group was significantly reduced. Although expression of miR-27a increased in compound C pretreated group, it did not reach the control group level. It was suggested that inhibition of AMPK expression could not completely relieve the inhibitory effect of GLP-1 on miR-27a expression. These results suggested that the AMPK signaling pathway is involved in the biological process of GLP-1, regulating miR-27a expression in MRC-5 cells; however, GLP-1 may also regulate the expression of miR-27a through other signaling pathways.
In this paper, we confirmed that high glucose promoted the expression of miR-27a, inhibited the expression of PPARγ, increased the expression of extracellular matrix-related genes, Col-IV and fibronectin, and extracellular matrix synthesis pathway related gene, NF-kB p65, and increased expression of differentiation and proliferation-related genes α-SMA and TGF-β1 in human embryonic lung fibroblasts. The expression of apoptosis-related gene caspase-3 was significantly decreased. miR-27a inhibitor reduced miR-27a expression and reversed all of the above process. It was suggested that miR-27a played an important role in the process of MRC-5 cell fibrosis induced by high glucose. We then treated MRC-5 cells with different concentrations of GLP-1 at different times, which suggested that the effect of GLP-1 on miR-27a expression was time- and concentration- dependent. GLP-1 may play its role via the energy center AMPK. Investigations using MRC-5 cells that were pretreated with AMPK antagonist and treated with GLP-1 suggested that the AMPK signaling pathway is involved in the biological process of GLP-1’s regulation of miR-27a expression in MRC-5 cells, and that GLP-1 may also regulate the expression of miR-27a through other signaling pathways.
Acknowledgments
Funding: This work was supported by grants from the National Science Foundation of Tianjin (16JCYBJC26800, 18JCYBJC93300, 18JCZDJC35500), Tianjin Health Commission Science and Technology Talent Cultivation Project (2010KZ89), and Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (2009DX02).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-869
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-869
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-869). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davis WA, Knuiman M, Kendall P, et al. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2004;27:752-7. [Crossref] [PubMed]
- Enomoto T, Usuki J, Azuma A, et al. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest 2003;123:2007-11. [Crossref] [PubMed]
- Zhao W, Chen GM, Sun B, et al. Effects of liraglutide on the expression of local renin-angiotensin system, transforming growth factor-β1 and collagen type III in pulmonary tissue of diabetic rats. National Medical Journal of China 2014;94:459-63. [PubMed]
- Zhao W, Jiang LJ. Effects of rosiglitazone treatment on TGF-β1/Smad signaling pathways in Otsuka Long-Evans Tokushima Fatty rat lungs. Acta Anatomica Sinica 2011;42:226-31.
- Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 2007;18:1227-38. [Crossref] [PubMed]
- Kang BY, Park KK, Green DE, et al. Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature. PLoS One 2013;8:e79503 [Crossref] [PubMed]
- Wang J, Song Y, Zhang Y, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res 2012;22:516-27. [Crossref] [PubMed]
- Zhao W, Song XC, Jiang LJ, et al. The changes of expression of α-smooth muscle actin in lung of spontaneous type 2 diabetes rats. China Medicine 2011;6:541-3.
- Miao XY, Gu ZY, Liu P, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides 2013;39:71-9. [Crossref] [PubMed]
- Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 2011;54:1214-23. [Crossref] [PubMed]
- Pitocco D, Fuso L, Conte EG, et al. The diabetic lung--a new target organ? Rev Diabet Stud 2012;9:23-35. [Crossref] [PubMed]
- García-Sancho Figueroa MC, Carrillo G, Pérez-Padilla R, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respir Med 2010;104:305-9. [Crossref] [PubMed]
- Weynand B, Jonckheere A, Frans A, et al. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999;66:14-9. [Crossref] [PubMed]
- Hyldgaard C, Hilberg O, Bendstrup E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir Med 2014;108:647-53. [Crossref] [PubMed]
- Forgiarini LA Jr, Kretzmann NA, Porawski M, et al. Experimental diabetes mellitus: oxidative stress and changes in lung structure. J Bras Pneumol 2009;35:788-91. [Crossref] [PubMed]
- Gumieniczek A, Hopkala H, Wojtowicz Z, et al. Changes in antioxidant status of lung tissue in experimental diabetes in rabbits. Clin Biochem 2002;35:147-9. [Crossref] [PubMed]
- Singh S, Bodas M, Bhatraju NK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol 2016;310:L837-45. [Crossref] [PubMed]
- Zhao W, Zhang H, Xie Y, et al. The pathological changes of ultrastructure in bronchial mucosa of type 2 Diabetic patients. Acta Anatomica Sinica 2006;37:685-8.
- Zhang H, Bai JW, Yu ZT, et al. The changes and its clinical significance of ultrastructure in lungs of type 2 Diabetic patients. Acta Anatomica Sinica 2005;36:656-9.
- Choi JH, Jin SW, Choi CY, et al. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J Agric Food Chem 2017;65:317-26. [Crossref] [PubMed]
- Calvier L, Boucher P, Herz J, et al. LRP1 Deficiency in Vascular SMC Leads to Pulmonary Arterial Hypertension That Is Reversed by PPARγ Activation. Circ Res 2019;124:1778-85. [Crossref] [PubMed]
- Liu J, Zhao W, Song XC, et al. Effects of Rosiglitazone on Transforming Growth Factor-β1 and its Signaling Pathway Genes Expressions in Pulmonary Tissue of Otsuka Long-Evans Tokushima Fattv Rats. Chinese Journal of Prevention and Control of Chronic Diseases 2012;20:271-3.
- Tang X, Wang Y, Guo X, et al. Effects of glucagon like peptide-1 treatment on the alveolar capillary basal lamina in Otsuka Long-Evans Tokushima Fatty rats. Journal of Peking University (Health Sciences) 2008;40:178-80. [PubMed]
- Li Z, Ni CL, Yao Z, et al. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism 2014;63:1022-30. [Crossref] [PubMed]
- Wu J, Liu S, Yuan ZW, et al. MicroRNA-27a Suppresses Detrusor Fibrosis in Streptozotocin-Induced Diabetic Rats by Targeting PRKAA2 Through the TGF-β1/Smad3 Signaling Pathway. Cell Physiol Biochem 2018;45:1333-49. [Crossref] [PubMed]


