Numerical simulation and analysis of midfacial impacts and traumatic brain injuries
Introduction
The maxillofacial region is an exposed part of the human body and, as such, is susceptible to injury due to its limited protective capability. Maxillofacial injuries are common in traffic accidents, and in boxing, sports, and other violent incidents (1-3). Compared with upper face and lower face, the middle face area is so special that it contains most complex structures, includes many bones that form an integrated bone complex by bone sutures. The midfacial bone is rigidly connected with the skull base by suture, so force can be transmitted directly to the brain in the event of midfacial impact, therefore midfacial injuries are often accompanied by craniocerebral trauma. Bellamy et al. (4) reported on 3,291 patients with midfacial fractures and found that 21.3% of them had intracranial injuries, of which 6.3% died. Zandi et al. (5) evaluated 2,692 inpatients with maxillofacial trauma and found the rate of head injuries associated with facial bone fractures was 23.3%. The most common associated head injury was concussion, followed by cerebral contusion and skull fractures. Some research has suggested that fracture of facial bones, especially bones that are in anatomic proximity to the cranium, was a marker for an increased risk of head injuries (5-8). The analysis of mechanical processes and responses to injury can help surgeons better diagnose unsuspected brain injuries.
The traditional models of craniofacial injuries mainly include animal models, human cadaver models and anthropomorphic test devices (9,10). Although human cadaver models have similar anatomical structures and biomechanical tissue properties as the live human body, they have been greatly limited due to poor reproducibility and ethical concerns. The structure of anthropomorphic test devices is usually simplified so that complex mechanical details cannot be obtained. With the development of computer science, the finite element (FE) analysis offers a cost-effective method for solving complex mechanical problems with complicated geometries of traumatic situations via numerical simulations in a virtual environment, and the method is widely utilized in medical research for the study of biomechanical mechanisms in the head region. Since the 1970s, human head modeling based on FE analysis, which is used to predict the head injury risk and final injury location, has been a research hotspot. The first FE head model was proposed by Ward (11) to simulate impact injury based on cadaver tests. Subsequently, several FE head models have been reported in the literature in recent decades. A typical high-quality FE head model was developed in 2013 by Wayne State University (12). The robustness of the model was checked by 35 loading cases based on cadaver mechanic experiments.
To date, studies of head biomechanics using the FE analysis method have mainly focused on impact injury, explosion injury, firearm injury and helmet design (13-15). Most of them concern the biomechanical mechanism of head injury sustained by pedestrians, motorcyclists and vehicle occupants in road accidents. The impact force in accidents often causes severe head injuries, such as fractures, contusions, and hematomas. The FE simulation on this type of injury is more focused on craniocerebral trauma instead of facial injury. Some researchers have already begun to investigate the association of craniocerebral trauma with facial injury. Tuchtan et al. (16) investigated the forces transmission to the skull in the case of mandibular impact. Tse et al. (17) focused on stress distribution on the skull and brain in nine cases of facial impact. Compared to the clinical phenomenon mentioned previously, midfacial fractures are often associated with craniocerebral injury. According to the authors' knowledge, there have been few FE analysis-based studies investigating the biomechanical mechanism between the two injuries.
In order to understand this kind of injury mechanism, this study develops a human FE model with skull, midfacial bones and detailed anatomical intracranial features. The aim is to simulate twelve collision scenarios, with three different forces respectively imposed on four common injury sites in the midface. It is anticipated that this will enable us to obtain more information about stress distribution on the brain after midfacial impact and will also aid in understanding the aetiopathogenesis of traumatic brain injury (TBI) after blunt facial skull impact. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-21-134).
Methods
FE model description
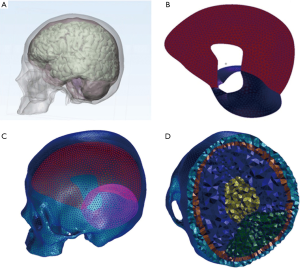
In our study, geometrical information of the human skull and brain were obtained from CT and MRI images of a healthy man (35 years old, 1.81 m, 74 kg). The data were saved as the DICOM (digital imaging and communications of medicine) standard and imported into the image processing software MIMICS 16.0 (Materialise, Leuven, Belgium). The bone and brain geometry were created from CT and MRI data respectively. Cerebrum, cerebellum and brain stem were reconstructed in MIMIC and the other main anatomical components (cerebrospinal fluid, falx and tentorium) were added in the meshing process. The meshing was produced using HyperMesh v10.0 (Altair HyperWorks, Troy, MI, USA). Twelve impact simulations mentioned above were performed in Patran/Nastran2012 (MSC.Software, USA) with an 8-cores workstation. The entire FE model is composed of 42,662 nodes and 223,152 linear tetrahedral elements. The total mass of the model is 4.132 kg. The bone, brain and cerebrospinal fluid (CSF) were developed using tetrahedral elements, and the falx and tentorium were developed using three-node shell elements (Figure 1). The thickness of the falx and tentorium was defined as 0.3 and 0.35 mm, respectively. All the FE model parts were defined as homogeneous and isotropic materials. The bone, CSF, falx and tentorium were modeled with linear elastic materials. CSF was modeled as a layer of a solid element with fluid-like properties and the coefficient of friction was 0.2. A viscoelastic law was implemented for the brain. This law was described by Herrmann and Peterson (18) in terms of relaxation shear modulus as defined by the following expression:
where G0, G∞ and β represent the short-time modulus, the long-time modulus and the decay constant, respectively. All the material properties that were acquired from published literature are shown in Table 1.

Full table
Numerical simulation
According to previous cadaver experiments (24-26), the middle section of the load curve is approximate to a half cycle of one sine. Because the collision time is very short (t=0–0.01 s), the impact force can be equivalent to transient load. According to the law of conservation of momentum: F·t=m·∆v, the transient impact load curve function is derived in the following steps:
In order to fit the experimental load curve, the width and amplitude of the function curve can be changed. Thus, we can make the impact load curve function as:
The collision duration is t (0–0.02 s) and the sine period is 0.002–0.008 s.
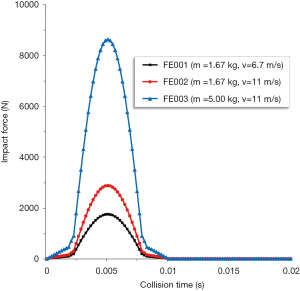
In our experiments, three different load forces were used to simulate common causes of injury seen in the clinic. The first two settings (FE001:m=1.67 kg, v=6.7 m/s; FE002:m=1.67 kg, v=11 m/s) were used to simulate boxing injury (27). Another setting (FE003:m=5 kg, v=11 m/s) was used to simulate car accident injury. According to the formula deduced above, the impact load curves of three cases were obtained (Figure 2). Four locations (nasal bone, frontal bone, margo infraorbitalis, zygomatic bone) in the midface were considered as common injury sites seen in the clinic (Figure 3). Therefore, twelve common impact scenarios were simulated.
Actually the process of craniocerebral impact injury includes several stages, including stress transmission injury in primary stage and inertial loading injury in later stage. Due to the large amount of calculation, It is very difficult to simulate the whole process. So in this paper, only the primary stage of the impact injury was simulated.
FE model validation
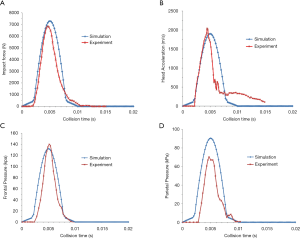
The model was validated by comparing it to the cadaver experimental results of frontal impacts conducted by Nahum et al. (24). In Nahum’s study, the foreheads of postmortem human subjects (PMHS) were impacted by a rigid impactor. To reproduce the same conditions as the experiment, the skull was rotated forward so that the Frankfort anatomical plane was inclined 45° to the horizontal. According to case 37 loading condition in the experiment, the model was frontally impacted by a 5.59 kg rigid cylindrical impactor with an initial velocity of 9.94 m/s. The results showed that impact force, head acceleration and frontal pressure were in good agreement with the cadaver experimental data (Figure 4A,B,C). The FE head model predicted the pressure was 90 kPa in the parietal region, slightly larger than the 70 kPa observed in the experiment condition (Figure 4D).
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients. This study was approved by the ethics committee of the General Hospital of Western Theater Command (No. 2018-12-03).
Results
An FE head model including midfacial bones, skull, brain, cerebrospinal fluid, falx and tentorium was successfully developed based on CT/MRI data. Von Mises stress fieldof the skull and brain were obtained through the simulation of the 12 cases. Stress distribution and potential injury regions were described and analyzed. The maximum stress of the bone and brain was measured and compared with the injury lowest limit. In order to analyze potential fracture regions of the facial bones fracture, the yielding limit was defined as 75 MP of Von Mises stress and the exceeded regions were marked blue on the 3-D models. According to the brain tissue tolerance thresholds in the literature, when Von Mises stress values exceed 20 or 30 kPa, mild TBI or severe TBI may occur, respectively. The two regions were marked blue and red respectively. In the following section, results of the simulations of the four different collision sites are analyzed.
Impact on the nasal bone (CS1, Figure 5
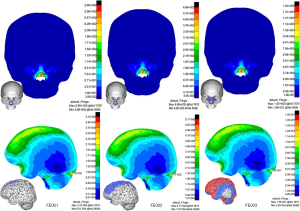
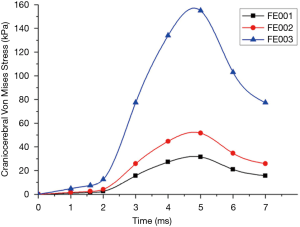
In FE001, when the speed was 6.7 m/s, the Von Mises stress peak value of bone reached 285 Mpa, and comminuted fractures probably occurred at the nasolateral and anterior nasal bone regions. Brain injury was very slight and only small areas of contusion occurred in the upper part of the frontal lobe. In FE002 when the speed reached 11 m/s, fractures were not limited to the nasal bone. Other fracture areas included the bilateral maxillary frontal processes and the frontal maxillary process. Mild TBI occurred at locations including the posterior frontal lobe, parietal lobe and anterior base of the brain where the stress ranged from 21.3 to 34.8 kPa. Stress concentrated at a small area of the posterior brain adjacent to the tentorium with the peak value of 51.7 kPa. In FE003 when simulating a car accident injury, possible fracture areas expanded including the entire nasal bone, maxillary frontal process, fontal maxillary process, lacrimal bone, ethmoid, and bilateral orbital floors. Severe TBI occurred at locations including the frontal lobe, parietal lobe, posterior occipital lobe, anterior temporal lobe and brainstem, suggestive of a fatal injury.
Impact on the zygomatic bone (CS2, Figure 6
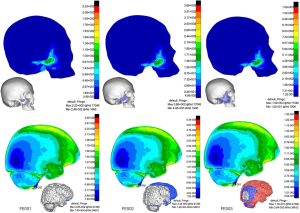
In FE001, microfracture might occur at the impact region of the zygomatic bone with a peak stress value of 223 MPa. In this low impact energy condition, the stress was concentrated in the anterior base of the brain and spinal cord, which are critical areas of brain functioning. Therefore, zygomatic impact is likely to be far more hazardous. In FE002, possible fracture areas expanded including the zygomatic frontal process and zygomatic arch. It was interesting that a region of stress concentration appeared at the zygomaticotemporal and the zygomaticomaxillary buttress after an interval of lower stress, which was consistent with the clinical zygomatic trauma. Mild TBI occurred at locations including the frontal lobe, anterior parietal lobe, and partial temporal lobe where the stress ranged from 20.9 to 32 kPa. In FE003, possible fracture areas included the zygomatic bone, zygomatic arch, temporal bone, lateral orbital surface, anterior maxillary orbital floor and skull base. Almost 70% of the lateral brain suffered severe TBI in this situation.
Impact on the infraorbital rim (CS3, Figure 7
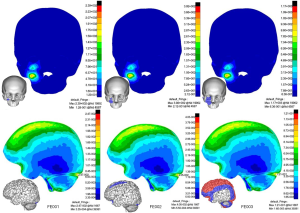
In FE001, fracture of bone and brain injury were very mild. In FE002, possible fracture areas extended to the anterior orbital floor and stress was concentrated at a small area of the posterior brain adjacent to the tentorium with the peak value of 40.5 kPa. In FE003, possible fracture areas included the anterior plate of maxillary, maxillary zygomatic process, orbital floor and lateral pyriform aperture margin. In FE003, TBI was similar to CS1, but less severe than CS1.
Impact on frontal bone (CS4, Figure 8
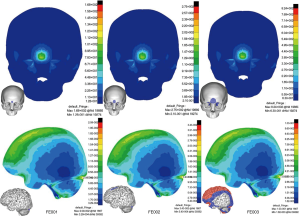
Fractures of bone and brain injury were very mild in both FE001 and FE002. In FE003, fracture areas extended to the bilateral orbital roof in addition to local impact sites. TBI was similar to CS1 and CS3. The peak stress of brain was located in the posterior brain adjacent to the tentorium.
Comparison of simulation results of four impact sites
The force transmission patterns of bones and stress distribution patterns of brain were very similar in regard to the same impact site. As the peak stress locations were different in the four impact situations, we chose stress value of brain at the frontal-parietal lobe junction as a comparison parameter (Figure 9). Forces on nasal bone caused the greatest Von Mises stress under the same load conditions. We extracted the peak stress curve of brain in regard to the impact on nasal bone (Figure 10). It showed that around 5 ms, the force of the brain reached its peak and then decreased slowly.
Discussion
Traumatic head injury seriously harms human health, and in most cases, a combination of impact force and acceleration of head is the cause of TBI. Impact forces are the cause of most focal injuries, and movement of the brain usually causes diffuse injuries. In clinical practice, the Glasgow Coma Scale (GCS) and severity of facial fracture are the basis for measuring maxillofacial injuries and traumatic brain injuries. Because the midfacial bones are connected directly to the skull, any force on the midface can be transmitted easily to the brain. Past studies have indicated that patients with midfacial fracture were significantly different from patients without midfacial fracture in severity of injury and were more likely to have a TBI (4,6,8). To investigate the relationship between the midfacial injury and the TBI, individual stress wave propagation paths from face to brain were presented in our study. Stress nephograms showed that force transmitted from the red areas to the blue areas. In the situation of impact on the nasal bone, stresses propagated in an anterior-posterior direction over the skull base from the bilateral orbital floors and ethmoid in the middle path. In the situation of impact on the zygomatic bone, stress waves travelled to the skull base through the zygomatic frontal process, frontal bone, maxilla, and sphenoidal orbit surface in the anterior path, as well as through the zygomatic arch and temporal bone in the posterior path. In the situation of impact on the infraorbital rim, stresses travelled from the orbital floor and maxilla to the skull base. In the situation of impact on the frontal bone, forces were transmitted directly to the brain. Stress concentration on the orbital roofs indicated that corresponding positions of the brain would be affected. The analysis of stress propagation can help us better understand and diagnose possible brain injury in the event of facial impact.
Our results showed that there was a linear relationship between the severity of TBI and the collision energy. TBI was aggravated with the increase of impact velocity and impact mass. In the simulation of boxing injuries, when impact velocity increased to 11 m/s, the TBIs were more severe, but most would be classified as mild injuries. But when impact mass increased to 5 kg, the TBIs were so significant that almost the entire brain suffered severe TBI. This phenomenon indicates that TBIs are more susceptible to the impact mass. Viano et al. (27) compared football impact injuries with boxing injuries caused by Olympic boxers and found that Olympic boxers deliver punches with high impact velocity but lower brain injury risks than that in football impacts because of a lower effective punch mass. This is consistent with our study.
In clinical practice, TBI is often accompanied by complex midfacial fractures, and the severity of TBI is also relevant to the location of the brain injury (28). For example, injuries in the brain stem or base of the brain are more hazardous than injuries in the frontal lobe. Several researchers have reported that TBI might occur in patients with facial fractures without any obvious signs on MRI or CT examinations (2,29,30). Most patients with non-neurological impairment do not need surgical treatment, but it does not mean that there’s no injury in the brain. So in these cases, the mechanism of the facial injury should be analyzed to determine whether there is a possible brain injury in adjacent important regions.
The location of TBI is directly related to the location of the impact site. We found the patterns of TBIs were very similar in the three situations of anterior-posterior direction impact, including impact on the nasal bone, frontal bone and infraorbital rim. In this kind of impact, the frontal lobe of the brain was directly affected, and the depth of propagation of the stress wave reached the deep level of the brain. And in all three impact scenarios, stresses were concentrated in the surfaces of the brain adjacent to the tentorium. In the case of lateral impact on the zygomatic bone, the stress concentrated in the anterior base of the brain and the spinal cord, which could be much more dangerous given the importance of these regions in brain function. The results were consistent with some previous studies. Zhang et al. (31) found that a lateral impact was more injurious than a frontal impact in previous experimental studies. Haug et al. (28) reported that the most severe intracranial injuries are found in zygomaticomaxillary fractures.
In our model CSF was modeled as a layer of a solid element with fluid-like properties so as to allow the brain to move relative to the skull during an impact. In all simulations, there were stress concentrations at the frontal lobe, parietal lobe and brain adjacent to the tentorium. It indicated that the intrinsic sharp structure of the skull could cause physical damage in the event of collision with the brain. Another interesting phenomenon was that the peak Von Mises stress value of the skull and brain under frontal impact was the lowest compared to the other three impact scenarios. This can be interpreted by the finding conducted by Huempfner-Hierl et al. (32) that the supraorbital arch is a structure which is able to carry loads from impacts and protects the skull and brain. Chang et al. (33) found that midfacial bones acted as a structure capable of absorbing considerable energy in order to protect the brain from impact. In this experiment, although the peak Von Mises stress value of brain was highest under nasal impact, which indicated more severe TBI, the stress value of the bone was also highest too. Due to the frangibility of nasal bones, fracture might occur causing impact energy to be absorbed. As we all know, facial bone is prone to fracture, due to its more cancellous bone content. When the head is injured, the appearance of facial bone fractures can offset some forces to protect the brain.
The FE model in this study has its limitations. The simulation does not take the neck into consideration during impact processes. Although studies found that for short duration impacts (0–6 ms) (20,34), the neck does not influence the kinematic head response, there is no doubt that the neck plays an important role in TBI during the latter stage of the collision. The model does not consider the buffering effect of boxing gloves and soft tissues. Also, the brain is defined as consisting of homogeneous and isotropic materials, which is inconsistent with the fact that the brain is anisotropic visco-hyperelastic (35). However, the analysis of correlation between midfacial injuries and TBIs provides useful information in the diagnosis of facial trauma.
Conclusions
In this study, a FE head model including midfacial bones, skull, brain, cerebrospinal fluid, falx and tentorium was successfully developed based on CT/MRI data. Twelve collision scenarios including four impact sites and three load forces were simulated. Results showed that there was a linear relationship between the severity of TBI and the collision energy. The location of TBI was directly related to the location of the impact site, and a lateral impact was more injurious to the brain than that of an anterior-posterior impact. The relative movement between the skull and brain could cause physical damage to the brain. The study indicated that the midfacial bones acted as a structure that was capable of absorbing energy and protecting the brain from impact. This information may assist surgeons to better understand and diagnose brain injuries accompanied by midfacial fractures.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-134
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-134
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-134). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients. This study was approved by the ethics committee of the General Hospital of Western Theater Command (No. 2018-12-03).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peden M, Scurfield R, Sleet D, et al. The world report on road traffic injury prevention. vol 4. World Health Organization; 2004.
- Jordan BD. Chronic traumatic brain injury associated with boxing. Semin Neurol 2000;20:179-85. [Crossref] [PubMed]
- Peskind ER, Brody D, Cernak I, et al. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry 2013;74:180-8. [Crossref] [PubMed]
- Bellamy JL, Mundinger GS, Reddy SK, et al. Le Fort II fractures are associated with death: a comparison of simple and complex midface fractures. J Oral Maxillofac Surg 2013;71:1556-62. [Crossref] [PubMed]
- Zandi M, Seyed Hoseini SR. The relationship between head injury and facial trauma: a case-control study. Oral Maxillofac Surg 2013;17:201-7. [Crossref] [PubMed]
- Joshi UM, Ramdurg S, Saikar S, et al. Brain Injuries and Facial Fractures: A Prospective Study of Incidence of Head Injury Associated with Maxillofacial Trauma. Journal of maxillofacial and oral surgery 2018;17:531-537. [Crossref] [PubMed]
- Lee KF, Wagner LK, Lee YE, et al. The impact-absorbing effects of facial fractures in closed-head injuries. An analysis of 210 patients. J Neurosurg 1987;66:542-7. [Crossref] [PubMed]
- Smith HL, Chrischilles E, Janus TJ, et al. Clinical indicators of midface fracture in patients with trauma. Dent Traumatol 2013;29:313-8. [Crossref] [PubMed]
- Gennarelli TA, Thibault LE, Adams JH, et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 1982;12:564-74. [Crossref] [PubMed]
- Yoganandan N, Maiman DJ, Guan Y, et al. Importance of physical properties of the human head on head-neck injury metrics. Traffic Inj Prev 2009;10:488-96. [Crossref] [PubMed]
- Ward CC, Thompson RB, editors. The development of a detailed finite element brain model. Stapp Car Crash Conference; San Diego, CA: SAE International, 1975.
- Mao H, Zhang L, Jiang B, et al. Development of a finite element human head model partially validated with thirty five experimental cases. J Biomech Eng 2013;135:111002 [Crossref] [PubMed]
- Chen Y, Miao Y, Xu C, et al. Wound ballistics of the pig mandibular angle: a preliminary finite element analysis and experimental study. J Biomech 2010;43:1131-7. [Crossref] [PubMed]
- Chang CH, Chang LT, Chang GL, et al. Head injury in facial impact--a finite element analysis of helmet chin bar performance. J Biomech Eng 2000;122:640-6. [Crossref] [PubMed]
- Sahoo D, Deck C, Yoganandan N, et al. Development of skull fracture criterion based on real-world head trauma simulations using finite element head model. J Mech Behav Biomed Mater 2016;57:24-41. [Crossref] [PubMed]
- Tuchtan L, Piercecchi-Marti M-D, Bartoli C, et al. Forces transmission to the skull in case of mandibular impact. Forensic Science International 2015;252:22-8. [Crossref] [PubMed]
- Tse KM, Tan LB, Lee SJ, et al. Investigation of the relationship between facial injuries and traumatic brain injuries using a realistic subject-specific finite element head model. Accid Anal Prev 2015;79:13-32. [Crossref] [PubMed]
- Herrmann LR, Peterson FE, editors. A numerical procedure for viscoelastic stress analysis. Orlando: Proceedings of the 7th Meeting of ICRPG Mechanical Behavior Working Group; 1968.
- Kleiven S, Hardy WN. Correlation of an FE Model of the Human Head with Local Brain Motion--Consequences for Injury Prediction. Stapp Car Crash J 2002;46:123-44. [Crossref] [PubMed]
- Willinger R, Taleb L, Kopp CM. Modal and temporal analysis of head mathematical models. J Neurotrauma 1995;12:743-54. [Crossref] [PubMed]
- Morrison B 3rd, Cater HL, Wang CC, et al. A tissue level tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp Car Crash J 2003;47:93-105. [Crossref] [PubMed]
- Turquier F, Kang HS, Trosseille X, et al. Validation study of a 3D finite element head model against experimental data. Journal of passenger cars 1996;pp.1912-1923. https://www.jstor.org/stable/44720909.
- Yoganandan N, Pintar FA, Zhang J, et al. Physical properties of the human head: Mass, center of gravity and moment of inertia. Journal of Biomechanics 2009;42:1177-92. [Crossref] [PubMed]
- Nahum AM, Smith R, Ward CC, editors. Intracranial pressure dynamics during head impact. Proc. of the Stapp Car Crash Confproc. of the Stapp Car Crash Conf; 1977:770922. https://www.sae.org/publications/technical-papers/content/770922/
- Cormier J, Manoogian S, Bisplinghoff J, et al. The tolerance of the frontal bone to blunt impact. J Biomech Eng 2011;133:021004 [Crossref] [PubMed]
- Cormier JM. Epidemiology and Biomechanical Analysis of Facial Fractures. Virginia Tech Virginia Polytechnic Institute and State, Blacksburg, Virginia, US, 2009.
- Viano DC, Casson IR, Pellman EJ, et al. Concussion in professional football: comparison with boxing head impacts--part 10. Neurosurgery 2005;57:1154-72; discussion 1172. [Crossref] [PubMed]
- Haug RH, Savage JD, Likavec MJ, et al. A review of 100 closed head injuries associated with facial fractures. J Oral Maxillofac Surg 1992;50:218-22. [Crossref] [PubMed]
- Salentijn EG, Peerdeman SM, Boffano P, et al. A ten-year analysis of the traumatic maxillofacial and brain injury patient in Amsterdam: incidence and aetiology. J Craniomaxillofac Surg 2014;42:705-10. [Crossref] [PubMed]
- Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet 2001;357:1391-6. [Crossref] [PubMed]
- Zhang L, Yang KH, King AI. Comparison of brain responses between frontal and lateral impacts by finite element modeling. J Neurotrauma 2001;18:21-30. [Crossref] [PubMed]
- Huempfner-Hierl H, Schaller A, Hierl T. Biomechanical investigation of the supraorbital arch - a transient FEA study on the impact of physical blows. Head Face Med 2014;10:13. [Crossref] [PubMed]
- Chang CJ, Chen YR, Noordhoff MS, et al. Maxillary involvement in central craniofacial fractures with associated head injuries. J Trauma 1994;37:807-11. [Crossref] [PubMed]
- Ruan JS, Khalil T, King AI. Human head dynamic response to side impact by finite element modeling. J Biomech Eng 1991;113:276-83. [Crossref] [PubMed]
- Sahoo D, Deck C, Willinger R. Development and validation of an advanced anisotropic visco-hyperelastic human brain FE model. Journal of the Mechanical Behavior of Biomedical Materials 2014;33:24-42. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)