
False-positive colloidal gold-based immunochromatographic strip assay reactions for antibodies to SARS-CoV-2 in patients with autoimmune diseases
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 (1), and the subsequent global spread of COVID-19 continues as evidenced by the rapid increase in the number of reported cases (2,3). Prompt infection control measures and public health surveillance to prevent the spread of COVID-19 rely on the early and accurate diagnosis of the disease. Thus, a rapid and accurate diagnostic test is needed to implement necessary quarantine measures and interventions (4,5). A failure to effectively diagnose COVID-19 may have serious implications not only for individual patients but also for public health.
Currently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid real-time reverse transcription polymerase chain reaction (RT-PCR) is the key method in COVID-19 diagnosis and treatment (6,7). However, the sampling site, sampling skills, and viral load can significantly impact the sensitivity of the nucleic acid test (8), and false-negative results have been reported in recent studies (9). Serological tests such as enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), colloidal gold–based immunochromatographic strip (ICS), and neutralization tests are effective in detecting an immune response to the virus and can be used in epidemiological investigation and population immunity assessment (10,11). Among them, the ICS assay is a rapid and convenient one-step immunochromatographic assay which is often used for rapid point-of-care screening of anti-SARS-CoV-2 immunoglobin M (IgM) and IgG. Detection of ICS anti-SARS-CoV-2 antibodies in blood samples can provide a quick and simple diagnostic method for suspected patients, with varied reported sensitivity 14 days after the onset of symptoms (24.5–100%) and specificity (90.1–100%) (12-15). However, serological testing is often limited by its potential cross-reactivity with antibodies caused by other coronaviruses and/or non-specific antibodies from past exposures (10,16).
Recently, we noted the occurrence of false-positive results in the ICS assay of sera from patients with autoimmune diseases (ADs). The immune system in patients with ADs produces a variety of auto-antibodies such as rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), and antinuclear antibodies (ANA), which may lead to cross-reactivity and false-positive results in serological testing (17). However, until now, the subject of false-positive results and cross-reactivity in the sera of patients with ADs remain unexplored. Patients with ADs are vulnerable to various viruses because their immunity is weakened by the use of immunosuppressants. Therefore, investigation of the causes for false-positive results is helpful in improving the clinical diagnosis and management of these patients. The false-positive results of immunoassays are frequently caused by the bindings of auto-antibodies, heterophilic antibodies and complements in serum to antibodies in the detection kit, but these bindings are often nonspecific and weaker than those of specific reactions. Some studies have found that interactions of this type can be reduced or eliminated by the addition to assay tests of a certain concentration of urea, which is a dissociating substance between nonspecific antigen-antibody reactions (18,19).
The ICS assay has been widely applied in the diagnosis of COVID-19. This study was conducted to determine the possible reasons for the false-positive results of ICS assay in AD patients and to investigate the effect of urea dissociation in reducing false-positive results. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/atm-20-6509).
Methods
Patients and samples
We measured SARS-CoV-2 IgM and IgG antibodies in 4 different cohorts: first group of 27 samples from 13 SARS-CoV-2 RT-PCR confirmed COVID-19 patients; a group of 120 healthy blood donors; a “disease control” group of 95 patients and a group of 135 AD patients. Serum samples from the participants were collected from the residual samples for the clinical routine, aliquoted, and then stored at –80 °C for further analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Peking University Third Hospital (No. YLS2020-171-01) and individual consent for this retrospective analysis was waived.
Serum samples from the COVID-19 group were obtained from 13 patients (the number of samples obtained from each individual patient ranged from 1 to 4 samples) at different time points during the disease course from February to April 2020. The patients were confirmed with COVID-19 by RT-PCR on nasopharyngeal swabs. To assess specificity, we collected 95 serum samples from 95 patients as control. These included (I) a disease control group of 60 patients with fever and/or respiratory infection but negative result of SARS-CoV-2 RT-PCR test in March 2020; (II) 13 samples from patients with influenza A or B virus infection (Wondfo rapid antigen test) in the period January to February 2019; (III) 10 Cytomegalovirus (CMV) IgM positive serum samples, 7 Rubella virus (RV) IgM positive serum samples and 5 Epstein-Barr virus (EBV) IgM positive serum samples collected from patients in March 2020. In addition, 120 serum samples from 120 healthy adults were evaluated to validate the performance of the ICS assay. The healthy controls were the hospital staff who undergone the yearly physical examination in October 2019, and had no complaints about health issues. All the healthy and disease controls were confirmed to be negative for COVID-19 by RT-PCR, and also confirmed without ADs.
The 135 AD patients were hospitalized at the Department of Rheumatology and Immunology, Peking University Third Hospital from January to November 2019, fulfilled the diagnostic criteria for ADs as defined by the American College of Rheumatology (ACR). The AD patients consisted of 7 with anti-synthetase syndrome (ASS), 7 with dermatomyositis (DM), 18 with systemic sclerosis (SSc), 14 with undifferentiated connective tissue disease (UCTD), 31 with rheumatoid arthritis (RA), 29 with systemic lupus erythematosus (SLE), and 29 with Sjögren syndrome (SS), none were infected by SARS-CoV-2 according to their clinical features, laboratory examinations and imaging manifestations.
ICS test and urea dissociation test
Two ICS test kits certified by the National Medical Products Administration were used. The first was used to detect total SARS-CoV-2 antibodies (Wondfo ICS, Wondfo Biotech Ltd., Guangzhou, China, lot no. W19500207), while the second was used to separately detect IgG and IgM antibodies (Innovita ICS, Innovita Biotech Ltd., Tangshan, China, lot no. 20200406). To perform each assay, a 10 µL serum sample and 2 drops of sample diluent were added to the sample pad, and the results in the “T” land “C” lines were observed after 15 min. The SARS-CoV-2 antibody in the sample bound first with the SARS-CoV-2 recombinant antigen labeled by colloidal gold, and was then captured by the anti-human-IgM (µ-chain specific) and IgG at the test line (T) position. A lack of the control line showing on the card indicated that the test was invalid. To investigate the effect of freezing on the results of these samples, we repeated the ICS test on 15 positive and 15 negative serum samples that had been frozen at –80 °C for 5 months and found that the results were the same as when previously measured.
A urea dissociation test of the ICS assay was performed for each SARS-CoV-2 antibody-positive sample according to the method proposed by Wang et al. with a small modification (18). Briefly, a 10 µL serum sample and 2 drops of sample diluent were added to the sample hole of the test card. When the liquid was about to reach the upper absorbent paper, 100 µL of phosphate-buffered saline (PBS) solution containing 6 mol/L urea was added into the sample hole, and the results were observed for 20 to 25 min. Duplication of the test was conducted to verify the results.
Autoantibody detection
ANAs were detected by IFA (Euroimmun, Lubeck, Germany). Serum IgG, IgA, IgM, and total RF was evaluated by a nephelometry assay (Immage 800, Beckman-Coulter, Brea, CA, USA), and the anti-CCP antibodies were assessed using an electrochemiluminescent immunoassay (ECLIA; Roche Diagnostics, Mannheim, Germany). Values above 20 IU/mL and 10 U/mL were considered positive for RF and anti-CCP, respectively. Other autoantibodies were detected by immunoblot assay (Euroimmun, Lubeck, Germany) including antibodies to Sjögren’s syndrome A 52 (SSA52), SSA, SSB, polymyositis-scleroderma antibodies (PM-Scl), proliferating cell nuclear antigen (PCNA), Smith antigen/nuclear ribonucleoprotein (Sm/RNP), ribosomal ribonucleoproteins (rRNP), Jo-1, scleroderma-associated antigen (Scl-70), double-stranded (ds) DNA, antihistone antibodies (AHA), anti-nucleosome antibody (ANUA), anti-centromere protein B antibodies (CENPB) and anti-mitochondrial antibody M2 (AMA-M2).
RT-PCR specific for SARS-CoV-2
SARS-CoV-2 viral RNA was detected in nasopharyngeal swabs of COVID-19 patients and controls. RNA was extracted from a 200 µL mixture using an automatic nucleic acid extraction instrument and magnetic bead method nucleic acid extraction kit (Nextractor 48, Seoul, South Korea). Detection of SARS-CoV-2 was performed with a commercial quantitative RT-PCR assay kit (Bojie, Shanghai, China). Primers and probes targeting SARS-CoV-2 open reading frame (ORF1a/b) and nucleocapsid (N) genes were used in the RT-PCR kit. The RT-PCR reactions were conducted in a thermal cycler (ABI7500 Real-Time PCR System, Applied Biosystems, USA) under the following conditions: 50 °C for 10 min for reverse transcription, followed by 95 °C for 5 min and then 40 cycles of 95 °C for 10 s, and 55 °C for 40 s. Samples with a cycle threshold (Ct) value below 38 were identified to be positive.
Statistical analysis
Statistical analyses were performed with SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Continuous variables with abnormal or normal distribution were expressed as the medians (25% quartile, 75% quartile) or mean (± standard deviation), respectively, and categorical variables are summarized as counts (percentages). A two-sample independent t test was used to compare the differences between two groups for continuous variables with normal distribution, and a nonparametric test (Mann-Whitney U tests) was used for continuous variables with abnormal distribution. We performed Pearson’s χ2 tests or Fisher’s exact test for the difference of proportions for categorical variables. Variables with a P value of <0.10 were included as candidates for the bivariable logistic regression to assess autoantibodies associated with false-positive results of SARS-CoV-2 antibodies. Missing data were excluded, and 2-sided P values <0.05 were defined as statistically significant.
Results
Detection of SARS-CoV-2 antibodies by the Innovita and Wondfo ICS assay
The healthy control group consisted of 42 male and 78 female with a mean age of 49.2 years (range 23–65 years). Specificity of Wondfo ICS kit in healthy control group was 97.5%, and was lower than that of Innovita ICS assay, which had a 100% specificity for IgM and 99.17% for IgG antibody (Table 1). The mean age of 13 COVID-19 patients was 54.3 (range, 25 to 73 years), with a majority being female (53.84%). Sensitivity was 74.07% for Wondfo compared to 70.37% for Innovita IgM and 66.67% for Innovita IgG. The diagnostic sensitivity was 68.18% for Wondfo compared to 63.64% for Innovita IgM and 59.09% for Innovita IgG within 14 days of symptom onset. The diagnostic sensitivity reached to 100% after 2 weeks of symptom onset for the two ICS rapid tests (Table 1). The disease control group consisted of 44 male and 51 female with a mean age of 51.2 years (range 19–72 years). Specificity of Wondfo ICS for 95 disease controls was 94.74% compared to 98.95% and 96.84% for Innovita SARS-CoV-2 IgM and IgG.
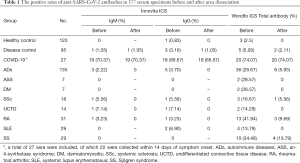
Full table
The AD disease group consisted of 17 male and 118 female with a mean age of 52.5 years (range 17–83 years). Thirty-six AD patients (26.67%) tested positive for total antibodies for SARS-CoV-2 according to the Wondfo ICS assay, while five AD patients (3.70%) tested positive for IgG and three patients (2.22%) tested positive for IgM using the Innovita ICS assay. The false-positive rate of total SARS-CoV-2 antibodies tested by Wondfo ICS was as high as 41.94% (13/31) in RA patients, followed by 34.48% (10/29) in patients with SS and 28.57% (2/7) in patients with ASS and DM. The positive rates of total antibodies in patients with SLE, SSc and UCTD were 13.79% (4/29), 16.67% (3/18), and 14.29% (2/14), respectively (Table 1).
Analysis of auto-antibodies and SARS-CoV-2 antibodies
While no auto-antibodies were detected in the serum samples of controls or COVID-19 patients, 1 or more auto-antibodies were detected in serum samples from the 135 AD patients. The AD patients were then divided into 2 groups according to the Wondfo ICS test results: the positive SARS-CoV-2 antibody group and the negative group, Table 2 lists the comparative results of auto-antibodies between the 2 groups. Age and sex were not statistically significantly different among AD patients positive or negative for antibodies [mean age, 55.3 (SD, 15.6) vs. 52.0 (SD, 15.4) years; 33/36 (91.67%) vs. 85/99 (85.86%) women].
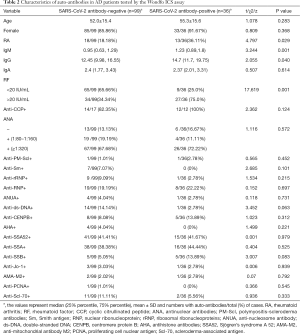
Full table
The difference in the distribution of RA patients in SARS-CoV-2 antibody-positive and antibody-negative groups was significant (P=0.029). The positive rate for SARS-CoV-2 antibodies in RA patients was higher than in non-RA patients [13/31 (41.94%) vs. 23/104 (22.12%)]. The median IgM and IgG levels were significantly lower in the SARS-CoV-2 antibody-negative group compared with the antibody-positive group (P=0.001; P=0.040). The percentage of elevated RF level (>20 IU/mL) was higher in the SARS-CoV-2 antibody-positive group compared with the antibody-negative group [27/36 (75.0%) vs. 34/99 (34.34%), P=0.001]; similarly, the percentage of lower RF level (<20 IU/mL) was lower in the antibody-positive group compared with the antibody-negative group [9/36 (25.0%) vs. 65/99 (65.66%), P=0.001]. Other auto-antibodies including anti-CCP, ANA, anti-SSA/SSB, anti-SSA52, anti-Sm, anti-rRNP, anti-RNP, ANUA, and anti-ds-DNA showed no significant difference between the SARS-CoV-2 antibody-positive and antibody-negative groups (Table 2).
In the bivariable logistic regression analysis, IgM, IgG, anti-SSB and anti-ds-DNA in sera of AD patients were not statistically significantly associated with antibody positivity (Table 3). In contrast, elevated RF (>20 IU/mL) was associated with antibody positivity [27/61 (44.26%) with elevated RF level vs. 9/74 (12.16%) with normal RF level; P=0.001], with an odds ratio of 4.671 (95% CI, 1.88–11.69).

Full table
Urea dissociation test of the ICS assay
The urea dissociation test of the Innovita ICS assay was conducted on antibody-positive sera from controls and AD patients (Figure 1). All the results of SARS-CoV-2 IgM and IgG analyses from the healthy controls were negative, whereas those from the confirmed COVID-19 patients remained positive (Table 1). One serum sample from a patient with fever and Escherichia coli bacteremia remained positive for SARS-CoV-2 IgM and IgG after urea dissociation test.

The urea dissociation test of the Wondfo ICS assay was conducted on 3 serum samples from healthy control, 5 from disease control and 36 from AD patients. The results of SARS-CoV-2 antibody analyses of 34 serum samples were negative, whereas those from the COVID-19 patients remained positive (Table 1). The specificity of the Wondfo ICS assay for the AD patients increased from 73.33% to 94.07% after the urea dissociation assay. In 27 serum samples positive for SARS-CoV-2 antibody and elevated RF, the SARS-CoV-2 antibody results were negative in 21 (77.78%) samples after urea dissociation. In addition, 7 of 9 serum samples with a normal RF level turned negative.
Discussion
Rapid and accurate testing for SARS-CoV-2 is needed to allow healthcare providers to make critical patient management decisions (20). Conventional serologic assays for viral‐specific IgM and IgG have been proposed to facilitate the diagnosis and infection monitoring of COVID-19, just as they were in the detection of severe acute respiratory syndrome coronavirus (SARS-CoV) (11,21,22). However, serological testing is often limited by its potential cross-reactivity and false-positivity caused by other pathogens and/or non-specific antibodies. The elimination of false-positive tests will greatly assist the serodiagnosis, epidemiological survey, and control of SARS-CoV-2, and an understanding of the causes of false-positive tests underpins this effort.
Our study showed that the specificity of the Wondfo ICS for AD patients was 73.33% compared to 97.78% and 96.30% for the Innovita ICS detecting SARS-CoV-2 IgM and IgG, respectively. Patients with ADs have a variety of auto-antibodies, which can cause false-positive results of immunoassay (17,18). Several studies have shown that RF is the most common of these (23-25), and this finding is supported by the results of our study. Our study showed that elevated RF levels (>20 IU/mL) were associated with antibody positivity using the Wondfo ICS assay, with an odds ratio of 4.671 (95% CI, 1.88–11.69). The positive rate of SARS-CoV-2 antibody was significantly higher in the elevated RF group than in the normal RF group (44.26% vs. 12.16%). The precise reason why sera containing elevated RF levels causes false-positive results when the Wondfo ICS is used is not clear and requires further research. Interactions may occur between RF and the various components used in ICS, such as colloidal gold-labeled recombinant SARS-CoV-2 antigen and anti-human IgM or IgG antibodies. Methods which may reduce the interference of RF include removal of the Fc fragment of the anti-human IgG antibody, dilution of samples, adding heat-denatured IgG to block the RF, and urea dissociation. The urea dissociation test is simple, easy to perform, and has been shown to be highly effective in reducing false-positive results of immunoassays associated with RF (18,26). In our study, we evaluated the effect of urea dissociation in reducing false positives of the ICS assay. Of the 27 sera with elevated RF and positive SARS-CoV-2 antibody tests, 21 turned negative, whereas the serum samples from COVID-19 patients were not affected, meanwhile, 7 of 9 serum samples with normal RF level turned negative. The false-positive results seen in the SARS-CoV-2 antibody were much less common in the Innovita ICS assay in comparison to the Wondfo ICS. Eleven serum samples positive for SARS-CoV-2 IgG or IgM tested negative after urea dissociation, indicating urea dissociation is useful in reducing non-specific interactions and false-positive results due to interfering factors other than RF. In this study, we also evaluated the influence of other auto-antibodies in patients with ADs on the detection of anti-SARS-CoV-2 IgM ang IgG. There was no significant difference in the distribution of ANA, anti-SSA/SSB, anti-SSA52, anti-CENPB, anti-RNP, anti-Scl-70 and anti-ds-DNA between the positive anti-SARS-CoV-2 IgM or IgG group and the negative group, which may indicate that the two test kits are less affected by these auto-antibodies.
While this study is a timely evaluation of the false positivity of Wondfo and Innovita ICS testing kits on COVID-19, it is worth noting some limitations. First, this is a retrospective study and some data were missing or not detected. For example, anti-CCP auto-antibodies were only detected in 29 serum samples. Second, the sample size was small, especially the number of serum samples from patients with COVID-19 and ASS. Finally, the interpretation of the results of the ICS assay was based on the visual observation of the T-line, and some light-colored test bands might have been interpreted as equivocal results. In our study, 30 out of 57 (52.63%) positive bands in control group were interpreted as weak immunoreactivity, 15 out of 57 (26.32%) positive bands of COVID-19 patients were weak. The accumulated experimental experience will help laboratory staff to interpret the band intensities in T-lines and report accurate results. Further well-designed prospective studies are needed to evaluate the efficacy of ICS assay in patients with different underlying conditions.
Conclusions
The Innovita ICS assay kit was found to be highly specific in both AD patients and controls. As a common auto-antibody in patients with ADs, elevated RF level (>20 IU/mL) was associated with antibody positivity using the Wondfo ICS assay. These false-positive results can be reduced by urea dissociation, and this should also be considered for the ICS assay.
Acknowledgments
We would like to thank LetPub (
Funding: This project was funded by National Major Scientific and Technological Special Project from Ministry of Science and Technology (No. 2017ZX09304012005 to LC).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/atm-20-6509
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6509
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6509). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Peking University Third Hospital (No. YLS2020-171-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- . The Lancet Infectious Diseases. COVID-19, a pandemic or not? Lancet Infect Dis 2020;20:383. [Crossref]
- Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020;9:313-9. [Crossref] [PubMed]
- Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill 2020;25:2003121 [PubMed]
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045 [Crossref] [PubMed]
- Cordes AK, Heim A. Rapid random access detection of the novel SARS-coronavirus-2 (SARS-CoV-2, previously 2019-nCoV) using an open access protocol for the Panther Fusion. J Clin Virol 2020;125:104305 [Crossref] [PubMed]
- Shirato K, Nao N, Katano H, et al. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis 2020;73:304-7. [Crossref] [PubMed]
- Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 2020;58:e00310-20. [Crossref] [PubMed]
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 2020;382:1177-9. [Crossref] [PubMed]
- Li D, Wang D, Dong J, et al. False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus 2: Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases. Korean J Radiol 2020;21:505-8. [Crossref] [PubMed]
- Pang J, Wang MX, Ang IYH, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med 2020;9:623. [Crossref] [PubMed]
- Wu HS, Chiu SC, Tseng TC, et al. Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan. Emerg Infect Dis 2004;10:304-10. [Crossref] [PubMed]
- Wu JL, Tseng WP, Lin CH, et al. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J Infect 2020;81:435-42. [Crossref] [PubMed]
- Van Elslande J, Houben E, Depypere M, et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect 2020;26:1082-87. [Crossref] [PubMed]
- Serrano MM, Rodriguez DN, Palop NT, et al. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol 2020;129:104529 [Crossref] [PubMed]
- Chen SY, Lee YL, Lin YC, et al. Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan. Emerg Microbes Infect 2020;9:2157-68. [Crossref] [PubMed]
- Maache M, Komurian-Pradel F, Rajoharison A, et al. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid-based western blot assay were rectified by the use of two subunits (S1 and S2) of spike for detection of antibody to SARS-CoV. Clin Vaccine Immunol 2006;13:409-14. [Crossref] [PubMed]
- Wang Y, Sun S, Shen H, et al. Cross-reaction of SARS-CoV antigen with autoantibodies in autoimmune diseases. Cell Mol Immunol 2004;1:304-7. [PubMed]
- Wang Q, Du Q, Guo B, et al. A Method To Prevent SARS-CoV-2 IgM False Positives in Gold Immunochromatography and Enzyme-Linked Immunosorbent Assays. J Clin Microbiol 2020;58:e00375-20. [Crossref] [PubMed]
- Lachaud L, Calas O, Picot MC, et al. Value of 2 IgG avidity commercial tests used alone or in association to date toxoplasmosis contamination. Diagn Microbiol Infect Dis 2009;64:267-74. [Crossref] [PubMed]
- Binnicker MJ. Emergence of a Novel Coronavirus Disease (COVID-19) and the Importance of Diagnostic Testing: Why Partnership between Clinical Laboratories, Public Health Agencies, and Industry Is Essential to Control the Outbreak. Clin Chem 2020;66:664-6. [Crossref] [PubMed]
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Woo PC, Lau SK, Tsoi HW, et al. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet 2004;363:841-5. [Crossref] [PubMed]
- Astarita G, Gutierrez S, Kogovsek N, et al. False positive in the measurement of thyroglobulin induced by rheumatoid factor. Clin Chim Acta 2015;447:43-6. [Crossref] [PubMed]
- Sutton BJ, Corper AL, Sohi MK, et al. The structure of a human rheumatoid factor bound to IgG Fc. Adv Exp Med Biol 1998;435:41-50. [Crossref] [PubMed]
- Duquerroy S, Stura EA, Bressanelli S, et al. Crystal structure of a human autoimmune complex between IgM rheumatoid factor RF61 and IgG1 Fc reveals a novel epitope and evidence for affinity maturation. J Mol Biol 2007;368:1321-31. [Crossref] [PubMed]
- Wang Q, Lei Y, Lu X, et al. Urea-mediated dissociation alleviate the false-positive Treponema pallidum-specific antibodies detected by ELISA. PLoS One 2019;14:e0212893 [Crossref] [PubMed]


