
Tumor necrosis factor receptor type 1-associated death domain (TRADD) regulates epithelial-mesenchymal transition (EMT), M1/M2 macrophage polarization and ectopic endometrial cysts formation in endometriosis
Introduction
Endometriosis is a gynecological non-malignant disease that affects 10–15% of women of childbearing age (1). It is manifested by the presence of extrauterine ectopic endometrial cells and stroma, which mainly occur in the peritoneal cavity (2). Endometriosis can lead to abdominal pain, dysmenorrhea, difficulty with sexual intercourse, and infertility, resulting in severe physical and psychological distress (3). Changes in many aspects of humoral and cellular immunity promote the pathogenesis of endometriosis (4-6). Pain induced by endometriosis can be treated by removing peritoneal lesions and ovarian cysts, or utilizing hormonal preparations, such as progesterone, gonadotropin-releasing hormone agonists, and oral contraceptives. However, despite these therapies, there is still a high recurrence rate (7). Therefore, more efficient treatment approaches are required.
The abnormal distribution of immune cells in the abdominal cavity of patients with endometriosis has been confirmed (8,9). Accumulating research has demonstrated that macrophages in the peritoneum show high activity in patients with endometriosis, and are thought to regulate and facilitate inflammation and disease maintenance (10). In ectopic patients with endometriosis, macrophages exhibit elevated activation and secretion, and synthesize a variety of pro-inflammatory factors, such as tumor necrosis factor (TNF)-α, interleukins (ILs), and macrophage-derived growth factors (11,12).
Macrophages are phagocytic cells in the immune system that are distributed in different tissues, and play an essential part in numerous diseases, such as inflammation and tumors (1). In addition, macrophages are highly heterogeneous and moldable cells that undergo transition from the M1 to M2 phenotype and vice versa, and are induced by specific effectors (13). Although uterine macrophages play an essential role in the function of the endometrium, the effects of human uterine macrophage activation and polarization on endometrial function maintenance and disease state development are poorly understood. Only several previously published studies have discussed the polarization of the macrophage phenotypes M1 and M2 in the human endometrium (14). A previous study indicated that human endometrial macrophages are mainly M2 macrophages (15). Only a small number of studies have evaluated the M1/M2 polarization of macrophages in patients with endometriosis (16).
Tumor necrosis factor receptor type 1-associated death domain (TRADD) protein is a TNF receptor-1 (TNFR1)-related signal transduction factor and a vital part of the TNFR1 compound. Both apoptotic and NF-κB pathways are activated by recruiting other constitutes of TNFR1 compounds to TRADD receptor (17). Studies on TRADD are generally focused on inflammatory responses and apoptosis (18,19). For instance, Ermolaeva et al. found that TRADD is a key part of TNFR1, toll-like receptor (TLR) 3, and TLR4 pathways and functions in TNF-induced apoptosis in a mice model (20). In the present study, we investigated the function of TRADD in endometriosis. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7866).
Methods
Clinical specimens
Clinical human normal adjacent tissues (normal group, n=20) and endometrial tissues (ectopic group, n=20) were attained from Beijing Obstetrics and Gynecology Hospital. Signed informed consent form was obtained from each patient prior to study participation. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The present study was approved by the Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital (No. SYXK (Jing) 2018-0003).
Cells and culture
Ectopic endometrial stromal cells (EcSCs) and eutopic endometrial stromal cells (EuSCs) were isolated from ectopic endometrial tissues with ovarian endometriosis and cultured in Dulbecco’s modified Eagle’s Media (DMEM; GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; GIBCO, USA). A human leukemia monocytic cell line THP-1 (ATCC, Manassas, VA, USA) was hatched in Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO, USA) with 10% fetal bovine serum (FBS) in an incubator containing 5% CO2 at 37 °C. To induce the differentiation of THP-1 cells into macrophages, cells were exposed to phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO, USA) for 72 h. After stimulation, non-adherent cells were discarded, and adherent cells were used as M0 macrophages.
Cell treatment
THP-1 cell-derived macrophages were treated with eutopic or ectopic endometrial homogenate from patients with endometriosis or endometrial homogenate from normal patients for 72 h. Experimental cells were then treated by 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich, USA) for 24 h and collected.
Cell transfection
Short-hairpin RNAs (shRNAs) directed against TRADD were ligated into the U6/GFP/Neo plasmid (GenePharma, Shanghai, China) as shRNA-TRADD1, shRNA-TRADD2, and shRNA-TRADD3. The U6/GFP/Neo plasmid carrying non-targeting sequences was used as the control. The full length of the TRADD coding sequence was cloned into pc-DNA3.1 (GenePharma, China), which are referred to as pc-DNA-TRADD. The empty pc-DNA3.1 were used as the control. Transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the standard protocol. ShRNA sequences for TRADD were listed below: sh-TRADD1, 5'-GGGUC AGCCU GUAGU GAAUTT-3', sh-TRADD2, 5'-TGCAG CTGCG ATTCT GCGGGC-3', sh-TRADD3, 5'-AAGTA AAACA GGAAT CAATCTTG-3'.
Western blotting
Total protein was isolated from EcSCs and EuSCs and quantified utilizing a bicinchoninic acid kit (Thermo Scientific Pierce, Rockford, IL, USA). Extracted proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and further transferred to a Poly (vinylidene fluoride) (PVDF) membrane. Primary antibodies of keratin (ab8068), TRADD (ab110644), N-cadherin (ab76057), vascular endothelial growth factor (VEGF; ab2350), CD206 (ab64693), CD40 (ab22469), matrix metalloproteinase (MMP)-14 (ab3644), E-cadherin (ab15148), Twist1 (ab50581), Vimentin (ab137321), IKK alpha (ab32041), NF-κB p65 (ab32536), NF-κB p-p65 (S536) (ab76302), p-MAPK (T202) (ab194776), MAPK (ab264269) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab128915) were incubated with membranes at 4 °C overnight; all primary antibodies were purchased from Abcam (Cambridge, MA, USA). After washing, the membranes were hatched with appropriate secondary antibody at room temperature. Bands carrying protein blots were visualized using a Tanon-5200 Image Analyzer (Tanon, Shanghai, China).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from normal uterine tissues, endometrial tissues, EcSCs, and EuSCs using TRIzol reagent (Invitrogen, USA). Extracted RNA was reverse transcribed into complementary DNA using the SuperScript III First Stand Synthesis System (Invitrogen, USA). The relative amount of mRNA was evaluated utilizing the equation 2–ΔΔCT as previous reports (19). Primer sequences of indicated genes were listed below. TRADD, sense primer: 5'-ACCAG CCTGG CAGAG GACTT-3', antisense primer: 5'-AGCAA TAGCC GCAGA AGGAA CC-3'. Keratin, sense primer: 5'-TGGCA GTGGT GGTGG TGGTT-3', antisense primer: 5'-GGCTG AAGAA GGCTC TGGTTGA-3'. E-cadherin, sense primer: 5'-TGATT CTGCT GCTCT TGCTG TT-3', antisense primer: 5'-CCTCT TCTCC GCCTC CTTCT TC-3'. N-cadherin, sense primer: 5'-TCATC CTGCT TATCC TTGTG-3', antisense primer: 5'-AGTCA TAGTC CTGGT CTTCT-3'. VEGF, sense primer: 5'-CCCAG GTCAG ACGGA CAGAA AG-3', antisense primer: 5'-AGCAG GTGAG AGTAA GCGAA GG-3'. CD40, sense primer: 5'-TGCCT TCCTT GCGGT GAAAGC-3', antisense primer: 5'-ACTCG TACAG TGCCA GCCTT CT. CD206, sense primer: 5'-GGCGG TGACC TCACA AGTATCC-3', antisense primer: 5'-TCATG GCTTG GTTCT CCACG AA-3'.
Wound healing assay
EuSCs and EcSCs were seeded onto a 6-well plate. When cells reached 90% confluency, the medium was removed and the wells were scratched by pipette tip. Plates were rinsed with phosphate buffered saline (PBS) to remove loose cells. Cells were then cultured in DMEM without FBS for 24 h. Images of the cells were taken at 0 and 24 h post-incubation, and the wound closure rate was calculated as follows: (1-scratch width at 24 h/scratch width at 0 h) ×100.
Transwell assay
After transfection, cells were seeded on the upper chamber of a 24-well plate. The lower chamber was filled with serum-free medium, and cells were incubated for 36 h. Unmigrated cells were discarded, and migrated cells were fixed and dyed with crystal violet (Beyotime Biotechnology, Shanghai, China). Stained cells were captured using an Olympus microscope (Olympus, Tokyo, Japan) at 5 random fields.
Interleukins (IL)-6 and IL-10 concentrations
Supernatants were collected, and IL-6 and IL-10 concentrations were determined utilizing an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific, Waltham, MA, USA).
Flow cytometric assay
THP-1 cells were blocked with anti-immunoglobulin antibody, collected with trypsin, and rinsed with PBS. THP-1 cells were stained by Phycoerythrin (PE)- Cluster of Differentiation 40 (CD40) and PE-Cluster of Differentiation 206 (CD206) at 4 °C under dark conditions for 30 min. THP-1 cells were detected by FACSAria (BD Biosciences, San Jose, CA, USA) using FACSDiva software (BD Biosciences, USA).
Rat endometriosis model
Twenty-four eight-week-old female Sprague-Dawley (SD) rats (240–260 g) were purchased from Lingchang Biotechnology (Shanghai, China). The present study was approved by the Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital (No. SYXK (Jing) 2018-0003). All animal handling and experimental procedures were in accordance with guideline of the Animal Experimental Ethnics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University for animal care and use. The rats were anesthetized with 4% isoflurane, then made a small incision at the center of abdomen. Excised the left uterine horn and placed it into saline solution. Dissected the endometrium from muscles, then cut into pieces (5 mm in each dimension). Made a subcutaneous pocket on the abdominal wall, then placed the uterine fragment in the pocket facing the abdominal muscle. Rats were then subcutaneously injected with 30 µg/kg benzoic estradiol immediately after surgery, then lasted for ten days. After successfully establishing of the rat endometriosis model, these rats were randomly divided into two groups (Endometriosis + TRADD and Endometriosis + EV). The rats of Endometriosis + TRADD group (n=8) received intraperitoneally injection with TRADD lentivirus for three times. The rats of Endometriosis + EV (empty vector) group (n=8) received intraperitoneally injection with EV lentivirus for three times. The sham group (n=8) underwent surgery without autotransplantation of endometrial tissues. The body weight and ectopic endometrial cyst volume were monitored every five days. The volume of ectopic endometrial cyst was calculated using the formula V = 1/2 (L × W2).
Immunohistochemistry (IHC)
The paraffin-embedded tissue samples were cut into 20 µm thick sections for IHC staining following the standard protocol. Tissue sections were deparaffinized by xylene and rehydrated by immersing in ethanol gradients (100%, 95%, 70% and 50%). Then tissues were rinsed in deionized water, and stained with hematoxylin for 5 min at room temperature.
Statistical analysis
All experiments were repeated 3 times. Results were performed as the mean ± standard deviation (sd), and evaluated utilizing GraphPad Prism 6.0 statistical software (GraphPad Software, La Jolla, CA, USA). P values were determined by analysis of variance, and P<0.05 was considered statistically significant.
Results
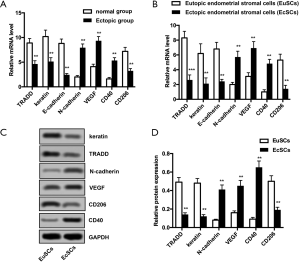
Abnormal expression of TRADD, keratin, E-cadherin, N-cadherin, VEGF, CD40, and CD206 in ectopic endometrial tissues and EcSCs
The expression of TRADD, keratin, E-cadherin, N-cadherin, VEGF, CD40, and CD206 was detected by qRT-PCR and Western blotting. As shown in Figure 1A,B, mRNA levels of TRADD, keratin, E-cadherin, and CD206 were significantly reduced in the ectopic and EcSCs groups (P<0.01 for both groups). In addition, mRNA levels of N-cadherin, VEGF, and CD40 significantly increased in both the ectopic and EcSCs groups (P<0.01 for both groups). The protein expression of these parameters was analyzed. Consisted with the results in Figure 1A,B, the protein expression of TRADD, keratin, and CD206 was significantly lower in the EcSCs group than that in the EuSCs group, but the protein expression of N-cadherin, VEGF, and CD40 was significantly increased in the EcSCs group compared with the EuSCs group (P<0.01 for both) (Figure 1C,D).
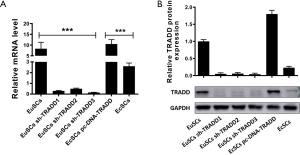
TRADD silencing in EuSCs or overexpression in EcSCs
To explore the function of TRADD in EuSCs and EcSCs, TRADD was silenced in EuSCs or overexpressed in EcSCs. qRT-PCR was performed to evaluate the efficiency of transfection in both cells. The mRNA level of TRADD1 was significantly reduced when TRADD was silenced in EuSCs (P<0.001), and the mRNA level of TRADD significantly increased with its overexpression in EcSCs (P<0.01) (Figure 2A). The protein expression of TRADD was detected using western blotting. TRADD downregulation could significantly decrease its protein expression in EuSCs (P<0.01), and TRADD upregulation resulted in increased expression in EcSCs (P<0.01) (Figure 2B).
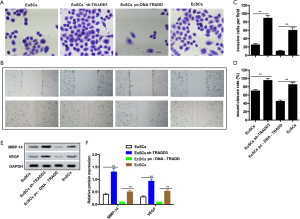
Overexpression of TRADD prohibits invasion and migration in EcSCs
The effects of TRADD on migration and invasion were determined in vitro, and the results of the transwell and wound healing assays are shown in Figure 3A,B,C,D. Cell invasion was clearly increased by silencing TRADD in EuSCs, but decreased by overexpression of TRADD in EcSCs (P<0.01). Similarly, the wound closure rate was significantly increased by TRADD knockdown in EuSCs, but was reduced by overexpression of TRADD in EcSCs (P<0.05). Figure 3E,F shows that TRADD silencing in EuSCs significantly increased the protein expression of MMP-14 and VEGF, and the high expression of TRADD in EcSCs significantly reduced these indexes (P<0.01). These data imply that TRADD silencing promotes migration and invasion in EuSCs, and TRADD overexpression reduced migration and invasion in EcSCs.
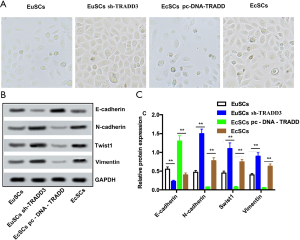
Overexpression of TRADD prohibits epithelial-mesenchymal transition (EMT) in EcSCs
EMT has been proven to be a key factor in migration and drug resistance (14). As shown in Figure 4A, morphological changes were observed; EuSCs were usually round; however, after transfection, most cells tended to be spindle shaped following TRADD silencing. In contrast, EcSCs were usually spindle shaped, and most cells tended to be round after TRADD overexpression. As shown in Figure 4B,C, TRADD3 silencing significantly increased the protein expression of N-cadherin, Twist1, and Vimentin in EuSCs (P<0.01). Conversely, TRADD3 knockdown clearly reduced E-cadherin expression in EuSCs (P<0.01). The expression of N-cadherin, Twist1, and Vimentin was significantly reduced by TRADD overexpression in EcSCs (P<0.05), and overexpression of TRADD significantly increased E-cadherin expression in EcSCs (P<0.05).
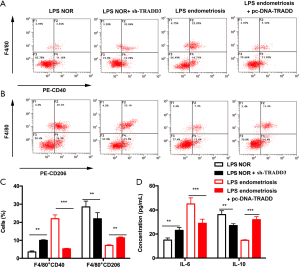
Upregulation of TRADD facilitated M1/M2 polarization in macrophages
THP-1 cell-derived macrophages were exposed to LPS for 24 h, followed by treatment with serum from patients with endometriosis or a normal endometrium for 72 days. After treatment, F4/80+CD40 positive cells of normal endometrial homogenization-treated THP-1-derived macrophages were notably elevated by TRADD silencing, whereas F4/80+CD40 positive cells of patients with endometrial homogenization-treated THP-1-derived macrophages were significantly reduced with TRADD overexpression (P<0.01 and P<0.001 for both). Additionally, TRADD inhibition clearly reduced the F4/80+CD206 positive cells of normal endometrial homogenization-treated THP-1-derived macrophages; however, F4/80+CD40 positive cells of patients with endometriosis and with endometrial homogenization-treated THP-1-derived macrophages were significantly increased with TRADD overexpression (P<0.01) (Figure 5A,B,C). In addition, the production of IL-6 and IL-10 was evaluated by ELISA. TRADD silencing clearly increased IL-6 production in normal endometrial homogenization-treated THP-1-derived macrophages, and TRADD overexpression significantly reduced IL-6 level in patients with endometrial homogenization-treated THP-1-derived macrophages (P<0.01 and P<0.001 for both). Conversely, IL-10 production was reduced by TRADD knockdown in normal endometrial homogenization-treated THP-1-derived macrophages, and TRADD upregulation increased IL-6 in endometrial homogenization-treated THP-1-derived macrophages (P<0.01 and P<0.001 for both) (Figure 5D).
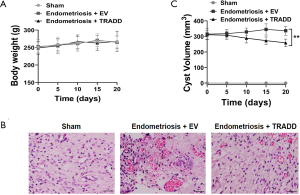
TRADD overexpression suppressed ectopic endometrial cysts formation in rat model
To evaluate if TRADD was involved in the progression of endometriosis in vivo, a rat model of endometriosis was established. As shown in Figure 6A, there was no significant difference of body weight between Sham, Endometriosis + EV or Endometriosis + TRADD group post-surgery. TRADD overexpression was realized by injection of TRADD expression lentivirus. IHC staining revealed that TRADD overexpression dramatically decreased ectopic endometrium formation compared with empty lentivirus control (EV) group (Figure 6B). The ectopic endometrium cyst volume of Endometriosis + TRADD group was also reduced compared with Endometriosis + EV group (Figure 6C). The above results indicated that TRADD overexpression suppressed ectopic endometrial cysts formation in vivo.
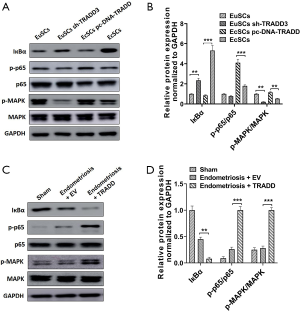
TRADD overexpression activated NF-κB and MAPK signaling in EcSCs and rat models
To explore the underling molecular mechanism of TRADD in endometriosis, activation of downstream pathways was evaluated by western blot. We were surprised to find that TRADD silencing reduced NF-κB and MAPK activation in EuSCs, and overexpression of TRADD increased NF-κB and MAPK activation in EcSCs (Figure 7A,B). TRADD silencing increased the expression of IкBα and reduced the phosphorylation of p-p65 and p-MAPK, while TRADD overexpression showed contrary effects (Figure 7A,B). In the rat model of endometriosis, TRADD overexpression significantly suppressed the expression of IкBα and upregulated the phosphorylation of p-p65 and p-MAPK, indicating that TRADD overexpression activated NF-κB and MAPK signaling in vivo (Figure 7C,D). Taken togther, TRADD regulated NF-κB and MAPK activation in EcSCs and rat models.
Discussion
Endometriosis is a chronic inflammatory disease that is associated with the production of cytokines and differential expression of immune inflammatory genes in the symptomatic peritoneal and ectopic endometrium (21,22). Endometriosis is not a cancer; however, is has several cancer-like characteristics, such as proliferation, migration, invasion, and anti-apoptosis (23). In the present study, we investigated the potential role of TRADD in the invasion, migration, EMT, M1/M2 polarization of THP-1-derived macrophages and a rat model of endimetriosis.
Migration and invasion play an essential role in the development of endometriotic cysts. Endometrial tissues are found to invade host tissues (24). According to Harada et al., VEGF promotes angiogenesis in endometrial implants (25). MMP expression in the ectopic endometrium from patients with endometriosis is different from that in normal patients. The protein expression of MMP-9 of ectopic endometrium from patients with endometriosis is notably higher than that of normal patients (26). It has been reported that MMP-9 is involved in invasion, and MMP-9 and VEGF are downstream molecules of the Wnt/β-catenin pathway (27-29). Therefore, the protein expression of MMP-14 and VEGF was measured. In the present study, the expression of MMP-14 and VEGF was promoted by TRADD silencing in EuSCs, while the expression of MMP-14 and VEGF was prohibited by TRADD overexpression in EcSCs, implying that TRADD can modulate migration and invasion in vitro.
Although endometriosis is not a malignant disease, its biologic behaviors, such as invasiveness, are similar to those of malignant tumors (30). Increasing research has indicated that invasiveness is related to the downregulation of E-cadherin, that is, epithelial cell phenotype damage is the result of the EMT cellular process (31). EMT is a complicated cellular process that transforms immobile epithelial cells into movable mesenchymal cells during embryonic growth, cancer, and physical responses to injury (32). Accumulating evidence has indicated that EMT may be associated with the migration and invasion in endometrial epithelial cells, and the process plays an essential part in the formation of endometriosis (33). EMT is characterized by reduced E-cadherin expression and elevated expression of mesenchymal markers, such as fibronectin and Vimentin (34). Additionally, existing research has revealed the key role of EMT in the occurrence and development of endometriosis (35). In the present study, we analyzed the expression of E-cadherin, N-cadherin, Twist1, and Vimentin. The data showed that that TRADD upregulation reduced migration, invasion, and EMT in EcSCs, whereas TRADD silencing had the opposite effect in EuSCs. These findings indicate that TRADD functions as an anti-EMT effector in endometriosis.
Previously published studies have implied that the disruption of the homeostasis of M1and M2 phenotypes may be involved in the pathogenesis of endometriosis (21,22). According to Bacci et al., endogenous macrophages are involved in tissue remodeling during the formation of endometriosis, and M1/M2 polarization is needed for the development of ectopic lesions (36). The M1/M2 polarization of macrophages is implied in the susceptibility and resistance to endometriosis; however, this is still controversial (1). A previous study found that, compared with the control group, the number of macrophages in the abdominal cavity of patients with endometriosis was remarkably promoted, but the proportion of CD163+ M2 cells was consistent (37). Bacci et al. found that peritoneal macrophages from patients with endometriosis and normal patients also express CD86 receptors (36). In the present study, we found that TRADD silencing increased the percentage of CD40 macrophages after eutopic homogenates under LPS exposure, while TRADD overexpression decreased the percentage of CD40 macrophages after ectopic endometrial homogenates under LPS exposure. Meanwhile, TRADD demonstrated opposite effects on the percentage of CD206 macrophages after the same treatments. Therefore, M1/M2 polarization was facilitated by TRADD in THP-1-derived macrophages, implying that TRADD silencing can prohibit the development of endometriosis to a certain degree. This was also supported by the production of IL-6 and IL-10.
In summary, we explored the effect of TRADD on migration, invasion, and EMT in endometriosis. The results implied that TRADD overexpression exerted inhibitory functions on migration, invasion and EMT in EuSCs and EcSCs. TRADD overexpression prohibited migration, invasion in EcSCs. In addition, TRADD overexpression promotes M1/M2 polarization in THP1 derived macrophages. In a rat endometriosis model, TRADD suppresses ectopic endometrial cysts formation. These effects may due to the activation of NF-κB and MAPK signaling by TRADD in EcSCs and rat models.
Acknowledgments
Funding: This work was supported by Beijing Excellent Talents Training Funding Project (No. 2018000021469G282).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7866
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7866
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7866). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Both human and animal experiments were approved by the Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital (No. SYXK (Jing) 2018-0003). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Signed informed consent form was obtained from each patient prior to study participation. All animal handling and experimental procedures were in accordance with guideline of the Animal Experimental Ethnics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University for animal care and use.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nie MF, Xie Q, Wu YH, et al. Serum and Ectopic Endometrium from Women with Endometriosis Modulate Macrophage M1/M2 Polarization via the Smad2/Smad3 Pathway. J Immunol Res 2018;2018:6285813. [Crossref] [PubMed]
- Mehedintu C, Plotogea MN, Ionescu S, et al. Endometriosis still a challenge. J Med Life 2014;7:349-57. [PubMed]
- Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261-75. [Crossref] [PubMed]
- Herington JL, Bruner-Tran KL, Lucas JA, et al. Immune interactions in endometriosis. Expert Rev Clin Immunol 2011;7:611-26. [Crossref] [PubMed]
- Kyama CM, Debrock S, Mwenda JM, et al. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol 2003;1:123. [Crossref] [PubMed]
- Matarese G, De Placido G, Nikas Y, et al. Pathogenesis of endometriosis: natural immunity dysfunction or autoimmune disease? Trends Mol Med 2003;9:223-8. [Crossref] [PubMed]
- Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261-75. [Crossref] [PubMed]
- Braun DP, Dmowski WP. Endometriosis: abnormal endometrium and dysfunctional immune response. Curr Opin Obstet Gynecol 1998;10:365-9. [Crossref] [PubMed]
- Jones RK, Bulmer JN, Searle RF. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update 1998;4:702-9. [Crossref] [PubMed]
- Takebayashi A, Kimura F, Kishi Y, et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am J Reprod Immunol 2015;73:221-31. [Crossref] [PubMed]
- Yang HL, Zhou WJ, Chang KK, et al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-beta. Reproduction 2017;154:815-25. [Crossref] [PubMed]
- Qiu XM, Lai ZZ, Ha SY, et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 2020;159:251-60. [Crossref] [PubMed]
- Tang MX, Hu XH, Liu ZZ, et al. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol 2015;112:73-80. [Crossref] [PubMed]
- Xu J, Liu D, Niu H, et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res 2017;36:19. [Crossref] [PubMed]
- Jensen AL, Collins J, Shipman EP, et al. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol 2012;68:374-86. [Crossref] [PubMed]
- Nie MF, Xie Q, Wu YH, et al. Serum and Ectopic Endometrium from Women with Endometriosis Modulate Macrophage M1/M2 Polarization via the Smad2/Smad3 Pathway. J Immunol Res 2018.6285813. [Crossref] [PubMed]
- Zhang X, Liu Z, Li C, et al. Grouper TRADD Mediates Innate Antiviral Immune Responses and Apoptosis Induced by Singapore Grouper Iridovirus (SGIV) Infection. Front Cell Infect Microbiol 2019;9:329. [Crossref] [PubMed]
- Dowling JP, Alsabbagh M, Del Casale C, et al. TRADD regulates perinatal development and adulthood survival in mice lacking RIPK1 and RIPK3. Nat Commun 2019;10:705. [Crossref] [PubMed]
- Shukla K, Sharma AK, Ward A, et al. MicroRNA-30c-2-3p negatively regulates NF-kappaB signaling and cell cycle progression through downregulation of TRADD and CCNE1 in breast cancer. Mol Oncol 2015;9:1106-19. [Crossref] [PubMed]
- Ermolaeva MA, Michallet MC, Papadopoulou N, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol 2008;9:1037-46. [Crossref] [PubMed]
- Ahn SH, Khalaj K, Young SL, et al. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril 2016;106:1420-31.e7. [Crossref] [PubMed]
- Khan KN, Kitajima M, Hiraki K, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril 2010;94:2860-3.e33. [Crossref] [PubMed]
- Lu Q, Huang Y, Wu J, et al. T-cadherin inhibits invasion and migration of endometrial stromal cells in endometriosis. Hum Reprod 2020;35:145-56. [Crossref] [PubMed]
- Xiong W, Zhang L, Yu L, et al. Estradiol promotes cells invasion by activating beta-catenin signaling pathway in endometriosis. Reproduction 2015;150:507-16. [Crossref] [PubMed]
- Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril 2001;76:1-10. [Crossref] [PubMed]
- Collette T, Maheux R, Mailloux J, et al. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum Reprod 2006;21:3059-67. [Crossref] [PubMed]
- Moon DO, Choi YH, Moon SK, et al. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol In Vitro 2010;24:1927-34. [Crossref] [PubMed]
- Kang H, Jang SW, Ko J. Human leucine zipper protein sLZIP induces migration and invasion of cervical cancer cells via expression of matrix metalloproteinase-9. J Biol Chem 2011;286:42072-81. [Crossref] [PubMed]
- Qu B, Liu BR, Du YJ, et al. Wnt/beta-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett 2014;7:1175-8. [Crossref] [PubMed]
- Kvaskoff M, Horne AW, Missmer SA. Informing women with endometriosis about ovarian cancer risk. Lancet 2017;390:2433-4. [Crossref] [PubMed]
- Xiong Y, Liu Y, Xiong W, et al. Hypoxia-inducible factor 1alpha-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum Reprod 2016;31:1327-38. [Crossref] [PubMed]
- Zhang M, Wang S, Tang L, et al. Downregulated circular RNA hsa_circ_0067301 regulates epithelial-mesenchymal transition in endometriosis via the miR-141/Notch signaling pathway. Biochem Biophys Res Commun 2019;514:71-7. [Crossref] [PubMed]
- Matsuzaki S, Darcha C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod 2012;27:712-21. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- Proestling K, Birner P, Gamperl S, et al. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod Biol Endocrinol 2015;13:75. [Crossref] [PubMed]
- Bacci M, Capobianco A, Monno A, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009;175:547-56. [Crossref] [PubMed]
- Itoh F, Komohara Y, Takaishi K, et al. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril 2013;99:1705-13. [Crossref] [PubMed]
(English Language Editor: R. Scott)


