
No diffuse intrahepatic biliary stricture after ABO-incompatible adult living donor liver transplantation using tailored rituximab-based desensitization protocol
Introduction
Rituximab (RTx) desensitization protocol in ABO-incompatible (ABOi) living donor liver transplantation (LDLT) offered a new paradigm shift beyond ABO blood barrier to obtain donor pool expanding (1). During the recent decade, high volume liver transplant centers mainly in east Asia have reported the feasibility and good outcome of RTx-based therapy without local infusion (2,3). However, diffuse intrahepatic biliary stricture (DIHBS) has been remained as an inevitable hurdle and reported 6.3–8% in recent reports (3-5) using RTx-based therapy. Therefore, various modifications in terms of plasmapheresis, splenectomy and intravenous immunoglobulin (IVIG) considering cost-effectiveness were proposed to overcome this hurdle. Here, in this study, we report the excellent outcome of ABOi adult LDLT without DIHBS using tailored desensitization protocol and compare it with that of ABO-compatible (ABOc) LDLT.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4703).
Methods
Study population and propensity score matching (PSM) (Figure 1)
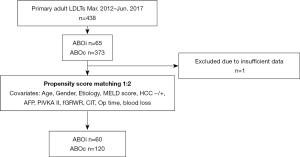
Between March 2012 and June 2017, we performed 65 cases (14.8%) of ABOi LDLTs in our center among 438 primary adult LDLTs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) at Seoul National University Hospital (SNUH) number “1910-036-1068”. Informed consent was waived by the IRB due to the retrospective study design. All methods employed in this study were performed in accordance with the relevant guidelines and regulations. One case was excluded due to insufficient data. We performed 1-to-2 PSM to extract ABOc cases. The propensity scores were generated using perioperative characteristics including age, sex, the etiology, the model for end-stage liver disease (MELD) score, the presence of hepatocellular carcinoma (HCC) or not, the level of alpha-fetoprotein (AFP) and PIVKA-II, final graft-to-recipient weight ratio (GRWR), cold ischemic time (CIT), recipient operative time, and recipient operative blood loss. Four cases of ABOi were excluded because of the mismatching of the propensity score. Finally, 60 ABOi cases and 120 ABOc cases were selected. The postoperative complication rates and overall survival (OS) and graft survival (GS) rates were then retrospectively compared between ABOc and ABOi cases.
Tailored desensitization and immunosuppression protocol for ABOi LDLTs
Our tailored desensitization protocol is shown in Figure 2. Reduced dose of RTx [300 mg/body surface area (BSA)] was administered around 3 weeks before LDLT, followed by 1 to 6 times of plasma exchange (PE) at 1 week before LDLT for a decrease of ABO isoagglutinin titer less than 1/16. If ABO isoagglutinin titer just before liver transplantation (LT) after PE was higher than 1/16, postoperative high-dose IVIG (0.8 g/kg/day of immunoglobulins were administered for 5 days from POD#1) and/or simultaneous splenectomy were selectively added. The splenectomy was sometimes skipped by surgeons’ decision. In emergent cases, a limited number of PE was done 48 hours after RTx administration. Immunosuppression was maintained with triple therapy; tacrolimus, steroids, and mycophenolate mofetil (MMF). During operation, methylprednisolone, 500 mg, was given intravenously. Postoperatively, methylprednisolone was tapered from 200 to 40 mg/day over 5 days and discontinued within 3 months after the operation. MMF (1 g/day) was given orally from POD1. The administration of tacrolimus was delayed and started orally on POD2 or 3 while renal function returned. The therapeutic trough levels (5–10 ng/mL) of tacrolimus were achieved within 7 days after transplantation.

Follow-up protocol for ABOi LDLTs
All patients who underwent LT had daily blood tests for checking liver function and tacrolimus level. Doppler sonography was performed to evaluate postoperative anastomosis until 6 days postoperatively. Computed tomography and routine liver biopsy were performed on day 7 postoperatively. ABO antibody titer was measured 2, 5 and 7 days after surgery.
Cytomegalovirus (CMV) antigenemia was followed up twice a week until discharge and then once a month until 3 months after LT. IV ganciclovir (GCV) was given preemptively if CMV antigenemia test shows equal or more than 5 positive cells/2×105 polymorphonuclear leukocytes.
All patients were discharged 2 weeks after surgery without any special complications and visited every week during the month of discharge.
Magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiography (ERC) were considered if patients were suspected of biliary complications with liver function abnormality such as elevation of alkaline phosphatase or gamma-glutamyl transpeptidase, instead of a routine examination. DIHBS was defined as bilateral multifocal stricture or diffuse necrosis of intrahepatic bile ducts in cholangiographic appearance according to our previous study (6).
Statistical analyses
The continuous values were compared by using the Mann-Whitney U-test, and categorical data were compared by using the chi-square test or Fisher’s exact test. The survival rates were estimated by the Kaplan-Meier method and compared in each group by the log-rank test. The significance threshold was P<0.05. PSM analysis was performed with SPSS, version 21.0 (SPSS Inc., Chicago, IL, USA). Other statistical analyses were performed with GraphPad Prism version 7.03 (GraphPad, San Diego, CA, USA).
Results
Desensitization results in ABOi LDLT
The results of our desensitization for ABOi were shown in Table 1. The timing of RTx administration was 19 days before LT (range, 4–33 days). Median frequency of pre-transplant PE was 3 times (range, 1–16). However, two cases with initially high titer (1:1,024) received 14 and 16 times of PE for 2 to 3 weeks among the initial series. The median IgM and IgG titers before LT were 2 (range, <1 to 64) and 16 (range, <1 to 512), respectively. There were 8 cases (12.5%) with longer days more than 3 weeks after rituximab among 64 ABOi cases due to various reasons (multiple PE during more than 1 week, delayed due to donors’ reasons, and so on). On the contrary, there were 3 emergent cases that the duration between RTx to LT was less than 1 week. Twelve recipients of ABOi series (18.8%) received simultaneous splenectomy and high-dose IVIG. Eight patients received perioperative high dose IVIG administration only.

Full table
The changes in isoagglutinin titer (IgG) was shown in Figure 3 according to the desensitization methods. There are 9 cases (20.5%) of rebound in no additional treatment group, 3 cases (37.5%) in IVIG only group, and 0 cases in IVIG + splenectomy group. However, there was no difference in biliary complication rate between desensitization method.

Basiliximab was added to the triple therapy for only the initial case in our ABOi series.
Patient characteristics
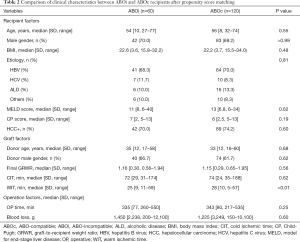
The clinical characteristics between ABOi (n=60) and ABOc (n=120) recipients after PSM are summarized in Table 2. There were no significant differences in clinical characteristics except for warm ischemic time (WIT) between ABOi and ABOc recipients. All recipients and donors were CMV IgG positive before LT.
Full table
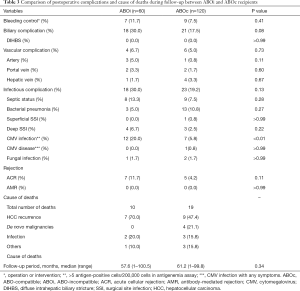
Postoperative complications and cause of deaths
The minimum follow-up was more than 3 years in all cases. The median length of follow–up for censored cases was 57.6 months (range, 1–100.5 months) in ABOi cases and 61.2 months (range, 1–99.8 months) in ABOc cases, without significant difference. Postoperative complications are shown in Table 3. There were no significant differences in vascular, biliary, and infectious complications and rejection. Biliary complication tended to be a little higher in ABOi cases. However, we have not encountered DIHBS related to ABO-incompatibility. Acute cellular rejection which was proven by biopsy occurred 7 cases (11.7%) in ABOi group and 5 cases (4.2%) in ABOc group, but that was not statistically significant. We also had no experience of antibody-mediated rejection (AMR) proven by protocol biopsy at postoperative day (POD) 7 in both groups. Twelve recipients of ABOi cases (20.0%) had CMV infection, which was significantly higher than ABOc cases. There was no CMV disease with symptoms in ABOi cases.
Full table
OS, GS, and biliary complication free survival
The OS and GS were shown in Figure 4. There were no significant differences in 1-, 3-, and 5-year survival rates of ABOi and ABOc groups (Figure 4A). Two patients in the ABOi group received re-transplantation for graft failure due to hepatic artery thrombosis (HAT), and intractable hyperbilirubinemia on POD 22 and 764, respectively (Figure 4B). The latter patient showed mild dilatation of intrahepatic duct and small gas-containing biloma outside of the liver (around cut surface of the graft) on CT scan at 8 months after LT. Percutaneous trans-hepatic biliary drainage (PTBD) showed the anastomosis stricture, leakage, and common bile duct stones, however, all intrahepatic duct was normal appearance. Multiple PTBD procedures and frequent infection associated with the infected biloma containing the detached artificial graft in the graft for draining of the middle hepatic vein (MHV) tributaries resulted in liver failure. This patient received deceased donor LT at POD764. The pathologic findings of the explant liver were compatible with biliary cirrhosis.

Finally, both recipients could be rescued. There were 10 deaths (16.7%) in ABOi group and 19 deaths (15.8%) in ABOc group. Most common cause of deaths was HCC recurrence (70.0% in ABOi, 47.4% in ABOc group) (Table 3).
Biliary complication (anastomosis leakage or stricture) free survival was compared (Figure 4C). The 1-, 3-, and 5-year biliary complication free survival rates were 81.4%, 69.5%, and 67.5% in ABOi group and 83.0%, 81.3%, and 80.0% in ABOc group, with no significant differences (P=0.11).
Discussion
ABO blood type barrier was used to be a big obstacle for LT. Local infusion therapy made a big footstep to better outcome (7,8), however, the survival rate was not satisfactory compared to ABOc LT (9). Second impact, RTx, anti-CD20 human chimeric monoclonal antibody, offered paradigm shift to breakthrough into even comparable survival (1,2,5,10-12). RTx is a potent immune-modulational drug to deplete B cell which produces an antibody leading to deleterious humoral rejection. According to Japanese multicenter study, after induction of RTx desensitization therapy, the incidence of acute type AMR such as hepatic necrosis decreased from 23.5% to 6.3% (3). Desensitization therapy by RTx obtains a consensus among many transplant centers and has completely replaced as fist place instead of local infusion therapy (1,5,11,13,14).
However, mild form of AMR-related complications is not rare even with RTx with/without PE protocol. Song et al. reported in their large single center experience that 7.1% of patients experienced AMR-related complications, all in the form of DIHBS (5). According to recent reports from Korean centers, its incidence was 6.4–7.2% (4,5). DIHBS is still a desperate complication that is refractory to interventions and often required re-transplantation. Therefore, an additional strategy is necessary to reduce the risk of AMR, especially in high-risk patients. However, the risk factor of DIHBS has not been identified yet. Even though Song et al. did not find any correlation between pre-LT or post-LT isoagglutinin titers and the occurrence of DIHBS (5), there are several reports to show the relationship of treatment non-responsive high isoagglutinin titers and AMR (3,15). We consider the patients with high titer even after RTx and PE therapy as a high-risk group. We applied additional strategy including IVIG and splenectomy in this high-risk group.
Even though a Japanese multicenter study concluded no significant additional effect of IVIG and raised the problem of cost (3). Several reports suggest IVIG was effective as a rescue of AMR and minimize the risk of AMR after ABOi LDLT (16-19). Therefore, considering potential effectiveness and cost-effectiveness, selective use of IVIG can be considered in the high-risk group. We compromised the use of IVIG and reached to optional use. So far, we never have AMR and DIHBS and this protocol is successful.
Splenectomy in ABOi LDLT is still a controversial issue. Kyoto group proposed that splenectomy does not offer additional immunological benefits with preoperative RTx (20). A recent report showed a trend that splenectomy is omitted with RTx B cell depletion except for the case of acute liver failure in that each center has different strategies (15,21). However, a splenectomy can prevent maturation of reappeared B cell after LT, theoretically. Therefore, it can be also considered selectively if there is no increased risk of infection or surgical complications. We never experienced splenectomy-associated major operative complications nor fatal infection. We balanced the risk and effect of RTx and applied selective splenectomy depending on the response of isoagglutinin titer to pre-LT PE as best. Our protocol enabled us to adjust desensitization therapy such as combined splenectomy, and IVIG according to the risk of AMR. That’s why we call our protocol “tailored”. The different outcomes in terms of AMR associated DIHBS of the previous studies from the literature review and this study were shown in Table 4.

Full table
Interestingly, there was higher proportion of rebound in IVIG only group and no additional treatment group than that in IVIG + splenectomy even though the pre-LT isoagglutinin titer was higher than the other groups. However, there was no difference in terms of the rate of biliary complications between groups.
We started ABOi LDLT program from March 2012. We have performed 132 cases until the end of 2019. The proportion of ABOi LDLT has been increasing from 3.6% in 2012 to 30.0% in 2019. Different from other series, so far, we have not encountered clinically significant AMR related complications by this tailored RTx based desensitization protocol. Thus, this satisfactory outcome may encourage the feasibility of our current protocol and support to use ABOi donor to expand the donor pool for LDLT candidates. However, additional randomized controlled study or PSM study between this tailored protocol and RTx-based protocol without IVIG nor splenectomy among ABOi patients are needed to confirm the usefulness of this tailored protocol.
Japanese multicenter study reported that more than 300 mg/BSA of RTx induction was the risk factor of CMV disease in univariate analysis (3). In our series, CMV infection was significantly higher in ABOi group than in ABOc group. However, there was no CMV disease with symptoms in ABOi group. Finally, CMV infection could be controlled by valganciclovir (VGCV) or GCV therapy in all ABOi cases. Therefore, our protocol was considered acceptable in terms of prevention for an infectious complication.
Although further investigation is needed such as a nationwide study to identify the risk factor and best protocol, our protocol showed satisfactory results.
To date, many transplant centers deploy RTx desensitization protocol, but each group has own modification based on their experiences. Usually, 375 mg/BSA is the normal dose in ABOi LT. We used a slightly reduced dosage of RTx as 300 mg/BSA for potential risk of infection, resulting in no severe infection after ABOi LDLT. Toki et al. investigated that low dose RTx (<375 mg/BSA) on splenic B cell population in recipients of ABOi kidney transplantation and almost complete depletion of CD20+ B cell from spleen (23). However, it is known that even in a single dose of 375 mg/BSA RTx could deplete almost all of B cell in the spleen and peripheral blood, the effect to lymph nodes is weak and memory B cell and plasma cell can survive in lymph nodes, at least 75% or more of whole peripheral lymphoid tissue (16,24,25). Egawa et al. reported that there was a tendency toward a higher incidence of AMR in patients treated with <300 mg/body compared with 500 or 375 mg/BSA (26). Therefore, we consider a reduced dose (300 mg/BSA) of RTx is a lower limit for balancing the prevention of AMR and severe infection.
About 70% of patients had HCC in our series. Because of B-cell depletion of RTx, there are concerns that the desensitization protocol might have a negative effect on HCC recurrence (27-29). However, our study focused on AMR related complications and infectious safety issues. Therefore, further study is needed to elucidate the oncological safety of our tailored protocol.
In conclusion, we haven’t experienced clinically significant AMR including DIHBS related to ABO-incompatibility in our series. There were no significant differences in infectious complications between ABOc and ABOi cases. Therefore, our RTx-based tailored desensitization protocol for ABOi LDLT was feasible and the outcome was acceptable in terms of OS and AMR associated complications.
However, additional randomized controlled study or PSM study between this tailored protocol and RTx-based protocol without IVIG nor splenectomy among ABOi patients are needed to confirm the usefulness of this tailored protocol.
Acknowledgments
We are particularly grateful for the assistance with statistics (propensity matching analysis) given by Dr. Seung-Young Oh.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4703
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4703
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-4703
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4703). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) at Seoul National University Hospital (SNUH) number “1910-036-1068”. Informed consent was waived by the IRB due to the retrospective study design. All methods employed in this study were performed in accordance with the relevant guidelines and regulations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Usuda M, Fujimori K, Koyamada N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation 2005;79:12-6. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol 2013;59:1215-22. [Crossref] [PubMed]
- Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant 2014;14:102-14. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg 2016;103:276-83. [Crossref] [PubMed]
- Song GW, Lee SG, Hwang S, et al. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Am J Transplant 2016;16:157-70. [Crossref] [PubMed]
- Lee HW, Suh KS, Shin WY, et al. Classification and prognosis of intrahepatic biliary stricture after liver transplantation. Liver Transpl 2007;13:1736-42. [Crossref] [PubMed]
- Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation 2002;73:1959-61. [Crossref] [PubMed]
- Umeshita K, Inomata Y, Furukawa H, et al. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res 2016;46:1171-86. [Crossref] [PubMed]
- Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 2008;47:143-52. [Crossref] [PubMed]
- Kim SH, Lee EC, Na BG, et al. Impact of ABO-incompatibility on hepatocellular carcinoma recurrence after living donor liver transplantation. Eur J Surg Oncol 2019;45:180-6. [Crossref] [PubMed]
- Yoon YI, Song GW, Lee SG, et al. Outcome of ABO-incompatible adult living-donor liver transplantation for patients with hepatocellular carcinoma. J Hepatol 2018;68:1153-62. [Crossref] [PubMed]
- Lee J, Lee JG, Lee JJ, et al. Results of ABO-incompatible liver transplantation using a simplified protocol at a single institution. Transplant Proc 2015;47:723-6. [Crossref] [PubMed]
- Yoshizawa A, Sakamoto S, Ogawa K, et al. New protocol of immunosuppression for liver transplantation across ABO barrier: the use of Rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc 2005;37:1718-9. [Crossref] [PubMed]
- Ikegami T, Yoshizumi T, Soejima Y, et al. Feasible usage of ABO incompatible grafts in living donor liver transplantation. Hepatobiliary Surg Nutr 2016;5:91-7. [PubMed]
- Ikegami T, Taketomi A, Soejima Y, et al. Successful ABO incompatible living donor liver transplantation in a patient with high isoagglutinin titer using high-dose intravenous immunoglobulin. Transplant Proc 2007;39:3491-4. [Crossref] [PubMed]
- Ikegami T, Taketomi A, Soejima Y, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation 2009;88:303-7. [Crossref] [PubMed]
- Morioka D, Sekido H, Kubota K, et al. Antibody-mediated rejection after adult ABO-incompatible liver transplantation remedied by gamma-globulin bolus infusion combined with plasmapheresis. Transplantation 2004;78:1225-8. [Crossref] [PubMed]
- Lee SD, Kim SH, Kong SY, et al. ABO-incompatible living donor liver transplantation without graft local infusion and splenectomy. HPB (Oxford) 2014;16:807-13. [Crossref] [PubMed]
- Kim JD, Choi DL, Kim SG, et al. Single-Center Experience of ABO-Incompatible Living-Donor Liver Transplantation with a New Simplified Intravenous Immunoglobulin Protocol: A Propensity Score-Matching Analysis. Transplant Proc 2016;48:1134-8. [Crossref] [PubMed]
- Raut V, Mori A, Kaido T, et al. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation 2012;93:99-105. [Crossref] [PubMed]
- Shen T, Lin BY, Jia JJ, et al. A modified protocol with rituximab and intravenous immunoglobulin in emergent ABO-incompatible liver transplantation for acute liver failure. Hepatobiliary Pancreat Dis Int 2014;13:395-401. [Crossref] [PubMed]
- Kim SH, Lee EC, Shim JR, et al. A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation. Liver Int 2018;38:932-9. [Crossref] [PubMed]
- Toki D, Ishida H, Horita S, et al. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int 2009;22:447-54. [Crossref] [PubMed]
- Egawa H, Ohmori K, Haga H, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl 2007;13:579-88. [Crossref] [PubMed]
- Llende M, Santiago-Delpín EA, Lavergne J. Immunobiological consequences of splenectomy: a review. J Surg Res 1986;40:85-94. [Crossref] [PubMed]
- Egawa H, Umeshita K, Uemoto S. Optimal dosage regimen for rituximab in ABO-incompatible living donor liver transplantation. J Hepatobiliary Pancreat Sci 2017;24:89-94. [Crossref] [PubMed]
- Miyagi S, Kawagishi N, Sekiguchi S, et al. The relationship between recurrences and immunosuppression on living donor liver transplantation for hepatocellular carcinoma. Transplant Proc 2012;44:797-801. [Crossref] [PubMed]
- Lee SD, Kim SH, Kong SY, et al. Kinetics of B, T, NK lymphocytes and isoagglutinin titers in ABO incompatible living donor liver transplantation using rituximab and basiliximab. Transpl Immunol 2015;32:29-34. [Crossref] [PubMed]
- Rummler S, Bauschke A, Baerthel E, et al. ABO-Incompatible Living Donor Liver Transplantation in Focus of Antibody Rebound. Transfus Med Hemother 2017;44:46-51. [Crossref] [PubMed]


