
Sentinel lymph node biopsy should be considered for clinically node-negative breast cancer regardless of BRCA1/2 mutation status
Introduction
Breast cancer is, by far, the most common malignancy in women worldwide (1). The stage at diagnosis and biological features help in determining prognosis. Surgery is always the primary treatment approach, and axillary lymph node staging is essential for evaluating the prognosis and planning the treatment. Completion axillary lymph node dissection (cALND) may lead to complications, including upper limb lymphedema, sensory numbness, and shoulder joint activity disorder, which could affect the patient’s quality of life (2). For this reason, sentinel lymph node biopsy (SLNB) has widely replaced cALND as routine axillary staging for patients with breast cancer with a clinically negative axilla according to the recommendations of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 Randomized Phase 3 Trial (3). Still, both Kim et al. (4) and Veronesi et al. (5) identified the safety of SLNB and recommended it should be the treatment of choice for patients who have early-stage breast cancer with clinically negative nodes.
Germline mutations in breast cancer susceptibility genes have become factors that influence the care of breast cancer patients. Surgeons are, therefore, required to integrate this information into surgical management decision-making (6). BRCA-associated breast cancer is the most common type of hereditary breast cancer, which can differ from sporadic breast cancer in both screening (7) and prevention. As Robson (8) reported increased both ipsilateral and contralateral risk of breast cancer for BRCA1/2 carriers, even breast-conserving therapy remains a relative contraindication to patients with breast cancer with BRCA1/2 predisposition according to the National Comprehensive Cancer Network guidelines version 2.2020 (9). Published study has reported worse outcome for patients with a BRCA1 or BRCA2 mutation compared with patients with sporadic breast cancer (10). Due to that the increased risk of breast cancer might result in easier metastasis of lymph node, it is still unknown that whether the possible worse prognosis with BRCA1/2 mutation would have effect on SLNB.
Several studies (11,12) have addressed the association between different BRCA1/2 mutation status and different strategies for surgical management of the breast (13,14). Practice guidelines have also addressed the prognostic impact of germline BRCA1/2 mutations on different surgical managements for the breast (i.e., breast-conserving therapy versus mastectomy) and have provided strategic suggestions (6,15). However, none involved the association between BRCA1/2 mutation status and the surgical management of the axilla.
Since cALND was the only option before SLNB was introduced in the early 1990s (16). Globally, SLN biopsies have been widely accepted only within the last ten years, but shorter in China only in the past five years. That is why none reported whether it is suitable that early breast cancer patients with BRCA1/2 mutation received SLNB. A study that enrolled patients between 1970 and 2003 did not include patients treated with SLN biopsies because these procedures were not practiced during this period (17). Even the American College of Surgeons Oncology Group Z0011 randomized trial (18) and Mansel et al. (19) did not stratify patients on the BRCA1/2 mutation status, likely following information on carrier status not being available from May 1999 to December 2004 (20). There is no such study in the Chinese population either.
According to the guidelines of the American Society of Clinical Oncology (ASCO), breast cancer patients with clinically negative axillary nodes are candidates for SLNB for axillary staging (21). However, the indications for SLNB are clinically node-negative breast cancer, additional factors that may also affect the decision to perform SLNB, which may influence the SLN involvement rate, and the prognosis (22). BRCA1/2 mutation status has not been one factor affecting SLNB for clinically node-negative breast cancer patients.
Genetic testing is not as frequent in China as it is in western countries. Chinese breast cancer patients with unknown BRCA mutation status receive SLNB if eligible because of the first presentation with clinically negative nodes. Among these, both BRCA1/2 carriers and non-carriers receive SLN biopsies. However, it is unknown whether BRCA1/2 mutation status would increase the SLN involvement rate and have a prognostic impact on breast cancer patients treated with SLN biopsy. Therefore, it remains debatable whether SLN biopsy, the current surgical management strategy of choice for axilla staging, is a safe and rational option for clinically node-negative breast cancer patients with germline BRCA1/2 mutations, especially in the Chinese population.
In this study, we enrolled breast cancer patients from the BRCA1/2 germline screening databases who were clinically node-negative on the first presentation and who received SLNB. We aim to investigate the association between the clinicopathological characteristics of patients, including BRCA1/2 mutation status, SLN involvement rate, and patient prognosis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5996).
Methods
Ethics
All the procedures performed in this study involving human participants were conducted following the ethical standards of the institutional and national research committees and with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. ZS 1655), and written informed consent was retrieved from all participants.
Study design and participants
Between January 2016 and April 2020, clinically node-negative primary breast cancer patients treated with conventional surgery from Peking Union Medical College Hospital and the Cancer Hospital of the Chinese Academy of Medical Sciences were retrospectively screened for BRCA1/2 germline mutations according to BRCA1/2 mutation test criteria. All patients who underwent initial SLNB were selected for this study. Among these, ALND was performed if micrometastases or macrometastases of SLNs were detected. Patients with a diagnosis of another malignant tumor and patients with simultaneous bilateral breast cancer were excluded from this cohort. The inclusion criteria were: (I) confirmed BRCA1/2 mutation status; (II) clinically node-negative primary breast cancer; (III) received initial SLNB. We excluded patients with a diagnosis of other malignant tumors, including gastric carcinoma, cervical carcinoma, or thyroid carcinoma, and simultaneous bilateral breast cancer.
Breast cancer patients were distributed to groups according to their BRCA1/2 mutation status and were classified as either BRCA1/2 mutation carriers or non-carriers. The primary endpoints were SLN involvement rate and breast cancer disease-free survival (DFS). SLN involvement rate was defined as the number of positive lymph nodes divided by the number of all lymph nodes during SLNB.DFS was defined as the first recurrence of the disease at a local, regional, or distant site or the diagnosis of contralateral breast cancer. The secondary endpoint is recurrence-free survival (RFS). The times to these endpoints were calculated from surgery (SLNB) to the first documented event. Breast cancer recurrence is categorized as a locoregional disease (tumors in the breast or ipsilateral supraclavicular, subclavicular, internal mammary, or axillary nodes) (18). Patients with no events were censored at the date of the last follow-up.
BRCA1/2 mutation testing
The criteria for genetic testing of BRCA1/2 mutation status included (I) triple-negative breast cancer (TNBC) (diagnosed ≤60 years of age); (II) breast cancer diagnosis ≤45 years of age; (III) breast cancer diagnosed at any age with at least one close blood relative with a family history of breast cancer, ovarian carcinoma, male breast cancer, prostate cancer, or pancreatic cancer.
Screening for BRCA1/2 mutations was performed by analyzing genomic DNA extracted from the patients’ peripheral blood and capturing targeted sequences followed by high-throughput sequencing. Quality control of the raw data was performed, followed by the removal of duplicate reads. Clean data were aligned to the hg19 reference genome using variants retrieved by GATK 4.0. ExAC further filtered through the variants and the database of the 1,000 genomes project. The filtered variants included untranslated region variants, intronic variants, splicing variants, and exotic variants. All deleterious mutations were confirmed by Sanger sequencing in duplicate. Pathogenic mutations and probable pathogenic mutations were defined as mutations that lead to a truncated protein or that had previously been reported to be associated with the disease.
Statistical analysis
Categorical variables are presented as frequencies and percentages for clinicopathological characteristics and were analyzed using Pearson’s chi-square test, Fisher’s exact test, and the continuity correction chi-square test. The univariate Kaplan-Meier method with log-ranking estimates was conducted to produce survival curves and to compare survival outcomes among different patient variables. Variables at or close to a value of P<0.05 in the univariate analysis, together with known critical clinical confounders (14,23), were used to perform multivariate Cox regression analysis to compare survival between different BRCA1/2 mutations. Variables included in the multivariable analysis include age at diagnosis (≤45 and >45 years), tumor size (≤2, 2–5, or >5 cm), TNM stage (stage 0, I, II, or III), estrogen receptor (ER) status (positive/negative), and Her-2 status (positive/negative). Systemic treatment for primary breast cancer was considered a variable, with the following categories: chemotherapy (yes/no), endocrine therapy (yes/no) (14), and surgical management of the breast. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
One hundred fifty-six clinically node-negative breast cancer patients underwent the first SLNB and were selected for this study. Of these patients, 31 of them were found as pathogenic BRCA1/2 germline mutation carriers (19.8%). These included 14 patients with a BRCA1 mutation (8.9%), 16 patients (10.3%) with a BRCA2 mutation, and one patient (0.6%) with both BRCA1 and BRCA2 mutations. A further 125 patients were classified as non-carriers. Five patients with a diagnosis of other malignant tumors, including gastric carcinoma (n=1), cervical carcinoma (n=2), and thyroid carcinoma (n=2), and one patient with simultaneous bilateral breast cancer were excluded. A further six patients were excluded because of incomplete medical records, and 11 subjects were lost to follow-up. 31 BRCA1/2 mutation carriers and 102 non-carriers were analyzed in the final cohort (Figure 1).
The analysis of clinicopathological characteristics according to the BRCA1/2 mutation status is shown in Table 1. BRCA1/2 mutation did not increase the SLN involvement rate for clinically node-negative breast cancer patients. This was because we did not observe a significant difference related to surgical management of the axilla, as 19.4% (6/31) of carriers had positive SLNs compared to 16.7% (17/102) of non-carriers (P=0.73). That meant that 6 (19.4%) carriers received ALND followed by SLNB, compared to 17 non-carriers (16.7%). The carrier group proved a tendency towards a slightly younger age than did the non-carrier group, although this difference was insignificant (P=0.38). Twenty-three carriers (74.2%) and 67 non-carriers (65.7%) were under 45 years of age when diagnosed with breast cancer. Interestingly, stage 0 and stage I were more frequent in the carrier group than in the non-carrier group (87.1% versus 59.8%, P<0.01), while stage II and III were more often present in non-carriers than in carriers (40.2% versus 12.9%, P<0.01). There is no significant difference in tumor size, histological type, estrogen receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor 2 (Her-2) status. The distribution of systemic therapy was similar between the two groups, including chemotherapy (P=0.19), radiotherapy (P= 0.64), and endocrine therapy (P=0.31).
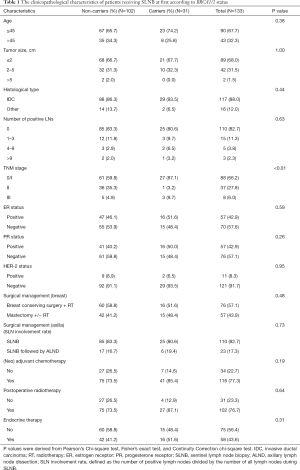
Full table
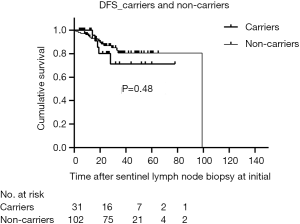
The median duration of follow-up for all patients was 28 months (range, 4–126 months), and 7.1% of patients were lost to follow-up. The RFS of carriers was like non-carriers (P=0.79) (Figure 2). In addition, there was no significant difference in DFS between the two groups (P=0.48) (Figure 3). For clinically node-negative patients who underwent SLNB at initial presentation, RFS was comparable between carriers and non-carriers [hazard ratio (HR) =0.82; 95% confidence interval (CI): 0.18–3.74; P=0.80]. After adjustment for age, tumor size, and TNM stage, the rate of RFS remained similar between groups (HR =0.89; 95% CI: 0.17–4.63; P=0.89). Likewise, differences in the HRs for RFS remained nonsignificant after additional adjustment for ER and Her-2 status (HR =0.86; 95% CI: 0.17–4.29; P=0.85) and for systemic treatment (HR =0.70; 95% CI: 0.14–3.52; P=0.66) (Table 2). After adjustment for surgical management of the breast, there was still no significant difference between the groups in RFS (HR =0.75; 95% CI: 0.14–3.89; P=0.73).
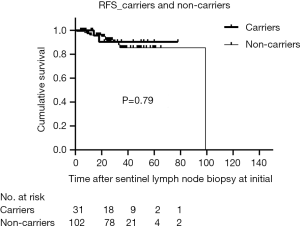

Full table
Although the results for DFS were in line with those for RFS (Table 3), some differences were observed. The HRs for breast cancer DFS are non-significantly higher both in the unadjusted analysis (HR =1.44; 95% CI: 0.52–3.97; P=0.48) and after adjustment for age, tumor size, and TNM stage (HR =1.77; 95% CI: 0.54–5.81; P=0.34). A similar pattern was observed upon added adjustment for ER and Her-2 status (HR =1.83; 95% CI: 0.57–5.82; P=0.30). Patients with BRCA1/2 mutations also showed a trend towards worse survival outcomes compared to non-carriers after adjustment for systemic therapy (HR =1.49; 95% CI: 0.45–4.97; P=0.51) and surgical management of the breast (HR =1.63; 95% CI: 0.48–5.54; P=0.43), although this did not reach the level of significance.

Full table
Discussion
SLNB has become the standard of care for primary treatment of early breast cancer, replacing ALND for clinically node-negative patients. However, many unresolved issues remain (22), which will still affect the decision-making of SLNB for clinically node-negative patients. Our study showed that BRCA1/2 mutation status did not increase the SLN involvement rate for the diagnosis of patients with clinically negative nodes (P=0.73); therefore, BRCA1/2 mutation cannot be used to prevent SLNB during the initial decision-making process. Several factors (22,24) increasing the SLN involvement rate still could affect the decision-making of SLNB for clinically node-negative breast cancer patients, including tumor size (25), multiple foci (26), imaging examination (27,28), and others. Completion ALND will be applied if SLN involved those factors that may bring unnecessary cALND with an increasing SLN involvement rate. So far, Giammarile et al. (29) suggested that axillary recurrence was associated with larger tumors. Simultaneously, Spillane et al. (30) reported that the identification rate for multiple breast cancers was like unifocal tumors, although there was a higher rate of positive SLN.
The false negative rate (FNR) of SLNB, different from the SLN involvement rate, is defined by doing a sentinel-node biopsy followed by a back-up axillary dissection to determine if there were additional positive nodes in the axillary dissection that were not seen on sentinel node biopsy (31). Krag et al. (32) reported that the SLNB technique is associated with a false negative rate of 5% to 10%. Several factors were reported increasing FNR of SLNB such as neoadjuvant chemotherapy (33) and mapping methods for SLNB (34). For the former, the FNR might be controlled through IHC staining combined with H&E staining for SLN and increasing the total numbers of SLN (17). For the latter, combination of two mapping methods for SLNB at least might take work including the use of blue dye tracer (e.g., isosulfan blue, methylene blue, and patent blue dye), use of radioisotope tracer, and use of indocyanine green (34). However, none of these earlier investigations have considered BRCA1/2 mutation status as a factor increasing FNR of SLNB. Further research may be designed investigating whether BRCA1/2 mutation status could increase the FNR of SLNB through comparing FNR between patients with or without BRCA1/2 mutation however in which all the patients should receive initial SLNB then followed by ALND. It is unrealistic and lake of operability in which SLNB was approved safely. In addition, those ways decreasing the FNR of SLNB still can be used for the BRCA carriers. Thus, To the best of our knowledge, it is enough to investigate the effect of BRCA1/2 mutation status on the SLN involvement rate for clinical node-negative breast cancer so that BRCA1/2 mutation will not affect the decision making of SLNB.
To enhance our findings, we also showed the prognostic impact of BRCA1/2 mutation status on the initial SLNB. DFS was comparable between carriers and non-carriers (P=0.48), findings that did not change after adjustment for clinicopathological characteristics, systemic treatment and surgical management of the breast (BCT versus mastectomy) (HR =1.63; 95% CI: 0.48–5.54; P=0.43). These results were consistent with those of the Z0011 trial, which also used DFS as the study endpoint (18). Additionally, RFS was in line between the groups (P=0.79). After adjustment for clinicopathological characteristics, and systemic treatment, it was still in line (HR =0.70; 95% CI: 0.14–3.53; P=0.66). Considering surgical management of the breast (BCT versus mastectomy) was an important confounding factor, it has been added into adjustment (HR =0.75; 95% CI: 0.14–3.89; P=0.73). These results were also in correspondence with the findings of the Dutch Randomized Controlled Multicenter Trial (BOOG 2013-08) as using RFS as the study endpoint (35). They reported that the 5-year regional RFS rate of SLNB was significantly non-inferior to ALND (99% versus 96%) for women with clinically node-negative T1–2 invasive breast cancer.
Both for non-adjusted and adjusted RFS and DFS, the carrier group HRs are below 1.0 for RFS but above 1.0 for DFS. These results were observed conversely to there being no significant differences between the groups. DFS events included metastasis and contralateral breast cancer; therefore, the difference in HRs between RFS and DFS might be because of the increased risk of contralateral breast cancer in carriers. These findings are supported by the findings of Kuchenbaecker et al. (36), who reported the risk of contralateral breast cancer increased in BRCA1/2 mutation carriers, especially among patients who carried the BRCA1 mutation.
We include different surgical management of the breast (BCT versus mastectomy) into adjusted analysis for the prognostic impact of BRCA1/2 mutations on SLNB. Surgical management related to breast cancer involves both breasts and axillae. BRCA1/2 mutation increased the risk of cancer in the breast; therefore, previous studies investigated whether BRCA1/2 mutations would affect the prognosis when different surgical management of breast (BCT vs. mastectomy) (14,37,38). Thus, in this study, different surgical management of breast was adjusted as an independent confounding factor for RFS and DFS so that both RFS and DFS would not be affected. Thus, it was necessary to consider whether different surgical management of breasts in the adjusted analysis for both RFS and DFS, especially for the former.
Although this is the first study to conclude that BRCA1/2 mutation does not increase involved SLN rate of initial SLNB or affect the prognosis of clinically node-negative breast cancer patients receiving initial SLNB, the study has limitations. The follow-up duration was insufficient. The small sample size and insufficient stratification by BRCA1 and BRCA2 mutation types are added limitations of this study. Even though, due to similar mechanism of both BRCA1 and BRCA2 in a common pathway of genome protection (39) and low frequency of BRCA1 or BRCA2 mutation (23), several studies (13,23) were still investigated without stratification by BRCA1 or BRCA2 mutation types. Only female patients were included for analysis. Thus, the findings may be applicable only for Chinese female patients with clinically node-negative breast cancer with BRCA1/2 mutations. Although the sample size is small, this is still exploratory research that can serve as the basis for prospective research.
Conclusions
In summary, this study suggests that BRCA1/2 mutations do not increase the SLN involvement rate of the initial SLNB. RFS and DFS for BRCA1/2 mutation carriers among Chinese women are equivalent to those of non-carriers. Thus, SLNB should be considered for clinically node-negative breast cancer regardless of BRCA1/2 status
Acknowledgments
The authors would like to express their gratitude to the study participants of the study. The authors also thank the Department of Breast Surgery from Peking Union Medical College Hospital and Department of Breast Surgical Oncology from the National Cancer Center for supplying the data used in this study.
Funding: The Fundamental Research Funds have supported this study for the Central Universities (3332019027), the National Natural Science Foundation of China (81802669 to JL), and the CAMS Initiative Fund for Medical Sciences (2016-I2M-1-001 to XW).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5996
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5996
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5996). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We state we have retrieved the Ethics Committee of Peking Union Medical College Hospital (No. ZS 1655) approval and have followed the principles outlined in the Declaration of Helsinki (as revised in 2013). Also, for investigations involving human subjects, informed consent has been retrieved from all the participants involved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wormann B. Breast cancer: basics, screening, diagnostics and treatment. Med Monatsschr Pharm 2017;40:55-64. [PubMed]
- Gillespie TC, Sayegh HE, Brunelle CL, et al. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 2018;7:379-403. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16. [Crossref] [PubMed]
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg 2010;251:595-600. [Crossref] [PubMed]
- Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol 2020;38:2080-106. [Crossref] [PubMed]
- Colin C, Doutriaux-Dumoulin I. Breast Cancer Screening in BRCA Mutation Carriers: Necessity of a Relevant Update of Mammographic Modalities. Radiology 2019;293:480-1. [Crossref] [PubMed]
- Robson M, Svahn T, McCormick B, et al. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: a clinic-based series. Cancer 2005;103:44-51. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) Clinical practices guidelines in oncology, Breast Cancer (2020). Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Feberury 5 2020.
- Huzarski T, Byrski T, Gronwald J, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 2013;31:3191-6. [Crossref] [PubMed]
- Biglia N, D'Alonzo M, Sgro LG, et al. Breast cancer treatment in mutation carriers: surgical treatment. Minerva Ginecol 2016;68:548-56. [PubMed]
- Pierce LJ, Phillips KA, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 2010;121:389-98. [Crossref] [PubMed]
- Nilsson MP, Hartman L, Kristoffersson U, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat 2014;147:571-8. [Crossref] [PubMed]
- van den Broek AJ, Schmidt MK, van 't Veer LJ, et al. Prognostic Impact of Breast-Conserving Therapy Versus Mastectomy of BRCA1/2 Mutation Carriers Compared With Noncarriers in a Consecutive Series of Young Breast Cancer Patients. Ann Surg 2019;270:364-72. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) Clinical practices guidelines in oncology, Genetic/Familial High-Risk Assessment: Breast and Ovarian (2019). Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed July 30 2018.
- Geng C, Chen X, Pan X, et al. The Feasibility and Accuracy of Sentinel Lymph Node Biopsy in Initially Clinically Node-Negative Breast Cancer after Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0162605. [Crossref] [PubMed]
- Neoadjuvant PD-1 Blockade in Resectable Lung Cancer; Nivolumab and Ipilimumab in Advanced Melanoma; Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma; Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma; Rapid Eradication of a Bulky Melanoma Mass with One Dose of Immunotherapy; Genetic Basis for Clinical Response to CTLA-4 Blockade; Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma; Nivolumab plus Ipilimumab in Advanced Melanoma; Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma; Hepatotoxicity with Combination of Vemurafenib and Ipilimumab. N Engl J Med 2018;379:2185. [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- He Z, Zhou Y, Wang F, et al. Clinical value of postoperative sentinel lymph node biopsy. Ann Transl Med 2019;7:683. [Crossref] [PubMed]
- Cao W, Xie Y, He Y, et al. Risk of ipsilateral breast tumor recurrence in primary invasive breast cancer following breast-conserving surgery with BRCA1 and BRCA2 mutation in China. Breast Cancer Res Treat 2019;175:749-54. [Crossref] [PubMed]
- Wiatrek R, Kruper L. Sentinel lymph node biopsy indications and controversies in breast cancer. Maturitas 2011;69:7-10. [Crossref] [PubMed]
- Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:561-4. [Crossref] [PubMed]
- Houvenaeghel G, Cohen M, Jauffret Fara C, et al. Sentinel lymph node-multicentric and multifocal tumors: a valid technique? Gynecol Obstet Fertil 2015;43:443-8. [Crossref] [PubMed]
- Nielsen Moody A, Bull J, Culpan AM, et al. Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: a systematic review and meta-analysis. Clin Radiol 2017;72:959-71. [Crossref] [PubMed]
- Galimberti V, Cole BF, Viale G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol 2018;19:1385-93. [Crossref] [PubMed]
- Giammarile F, Alazraki N, Aarsvold JN, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging 2013;40:1932-47. [Crossref] [PubMed]
- Spillane AJ, Brennan ME. Accuracy of sentinel lymph node biopsy in large and multifocal/multicentric breast carcinoma--a systematic review. Eur J Surg Oncol 2011;37:371-85. [Crossref] [PubMed]
- Edwin RF, Jiping W, John B, et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002;95:681-95. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8. [Crossref] [PubMed]
- Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg 2016;263:802-7. [Crossref] [PubMed]
- Li H, Jun Z, Zhi-Cheng G, et al. Factors that affect the false negative rate of sentinel lymph node mapping with methylene blue dye alone in breast cancer. J Int Med Res 2019;47:4841-53. [Crossref] [PubMed]
- van Roozendaal LM, Vane MLG, van Dalen T, et al. Clinically node negative breast cancer patients undergoing breast conserving therapy, sentinel lymph node procedure versus follow-up: a Dutch randomized controlled multicentre trial (BOOG 2013-08). BMC Cancer 2017;17:459. [Crossref] [PubMed]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. [Crossref] [PubMed]
- Schmidt MK, van den Broek AJ, Tollenaar RA, et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Garcia-Etienne CA, Barile M, Gentilini OD, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol 2009;16:3380-7. [Crossref] [PubMed]
- Roy R, Chun J, Powell SN, et al. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68-78. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)



