Novel inflammation-based prognostic nomograms for individualized prediction in hepatocellular carcinoma after radical resection
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer, and the third-leading cause of cancer-related mortality globally (1). At present, surgical resection is one of the most effective treatments with curative potential (2). However, because of the high incidence of tumor recurrence, long-term survival, the outcome after surgery is unsatisfactory and far from homogenous (3,4). Therefore, the stratification of these patients for individualized evaluation and follow-up after surgery is vitally important.
There is no consensus regarding the optimal tool for prognostic evaluation. The commonly used staging systems, including the 8th American Joint Committee on Cancer (AJCC) and Barcelona Clinic Liver Cancer (BCLC), have been proposed for risk stratification (5,6). However, these staging systems cannot provide an accurate prediction of the outcomes of individual patients, which various factors influence, including general condition, aggressive tumor characteristics, and systemic inflammation.
Inflammation is a prominent hallmark of cancer (7). There is increasing evidence to support the association between systemic inflammatory response and poor survival in various malignancies (8). Inflammation-related indexes, such as the neutrophil-to-lymphocyte ratio (NLR) (9), lymphocyte-to-monocyte ratio (LMR) (10), neutrophil times γ-glutamyl transpeptidase-to-lymphocyte ratio (NrLR) (11), platelet-to-lymphocyte ratio (PLR) (12), prognostic nutritional index (PNI) (13), γ-glutamyl transpeptidase-to-platelet ratio (GPR) (14), and systemic immune-inflammation index (SII) (15), have been reported as independent risk factors for prognosis in HCC patients after liver resection. Several predictive models based on inflammation-related indexes have been constructed, but mostly only focus on one or two inflammation-related indexes (16-18).
In this study, we used a large patient cohort to develop and validate novel nomograms incorporating clinical parameters and systemic inflammation to predict the long-term outcome for HCC patients after radical resection.
We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1919).
Methods
Patients
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (No. EHBHKY-2019-11-009). We obtained informed consent from each patient for their data to be used for research purposes. We reported this study according to the TRIPOD guidelines (19).
Between January 2008 and December 2014, data of patients with HCC who underwent primary hepatectomy at Eastern Hepatobiliary Surgery Hospital were prospectively collected and retrospectively analyzed.
The inclusion criteria were: (I) no extrahepatic metastasis; (II) no macrovascular invasion; and (III) R0 resection, which was defined as complete resection of the tumor with negative margins (20). Patients who underwent preoperative anticancer treatments, perioperative death, received palliative tumor resection, had a history of another type of cancer, had incomplete pathological data, or were lost to follow-up within 60 days after, it excluded surgery from the analysis.
The training cohort comprised eligible patients treated at Eastern Hepatobiliary Surgery Hospital between 2008 and 2012, while the validation cohort comprised patients who received treatment between 2013 and 2014.
Clinicopathologic variables and follow-up
Blood samples were obtained within 14 days before surgery for routine laboratory tests for liver function and blood cells. The formulas and cutoff values of NLR, LMR, NrLR, PLR, PNI, GPR, and SII are shown in Table S1.

Full table
We followed up with the patients once every three months for the first two years after discharge from the hospital and every three to six months after that. The follow-up program included liver function, serum AFP level, and an imaging study such as abdominal ultrasonography, contrast-enhanced abdominal computed tomography (CT), and abdominal magnetic resonance imaging (MRI). We based the diagnosis of recurrent HCC on CT and/or MRI and elevated AFP levels.
The end-points of the study were overall survival (OS) and recurrence-free survival (RFS). OS was defined as the interval between the date of surgery and the date of patient death or last follow-up. RFS was defined as the interval between the date of surgery and the date of last follow-up or death. The follow-up on 31 October 2019 was censored.
Statistical analysis
Categorical variables were presented as n (%) and compared using the chi-square test or Fisher’s exact test. The mean (standard deviation, SD) was obtained for continuous variables with normal distribution and compared using Student’s t-test. In contrast, the median (interquartile range, IQR) was obtained for continuous variables with non-normal distribution and compared using the Mann-Whitney U test. We identified the optimal cutoff value for these inflammation-related indexes using the “surv_cutpoint” function from the “survminer” R package.
All statistical tests were two-tailed, and P<0.05 represented statistical significance. All statistical analyses were performed with R version 3.5.2 (http://www.r-project.org/). The R packages of “rms”, “stdca”, “timeROC”, “CsChange”, “DynNom”, “survminer”, and “survival” were used in this study.
All factors with P<0.05 in univariate Cox regression were selected for multivariate Cox regression. The multivariate Cox regression was performed by a stepwise backward selection of variables. The nomograms were built based on the results of multivariate Cox regression in the training cohort.
Risk groups were generated by the previously reported cutoffs (50th and 85th percentile) of the score calculated by the nomogram (21). Kaplan-Meier analysis of each risk group was plotted for each cohort.
We assessed the performances of the nomograms according to discrimination, calibration, and clinical usefulness. Model discrimination was measured by C-index and time-dependent areas under the receiver operating characteristic curve (time-dependent AUC) (22). Model calibration was measured by the calibration curve. Clinical usefulness was measured by decision curve analysis (DCA) (23). The nomograms were also compared with the 8th AJCC, BCLC, and CNLC staging systems in each cohort (24).
Results
Clinicopathological features and inflammation-related index
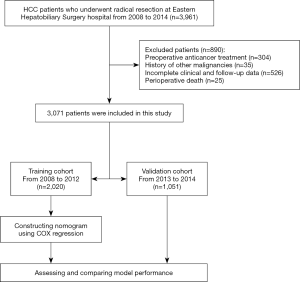
In total, 3,961 HCC patients underwent radical resection during the study period. Three thousand seventy-one patients who met the inclusion criteria were included in this study. In comparison, 890 patients were excluded for preoperative anticancer treatment (n=304), a history of other malignancies (n=35), incomplete clinical or follow-up data (n=526), or perioperative death (n=25). Data recorded from 2,020 patients treated between 2008 and 2012 formed the training cohort, and data recorded from 1,051 patients treated between 2013 and 2014 formed the validation cohort. The flow chart of this patient selection is shown in Figure S1.
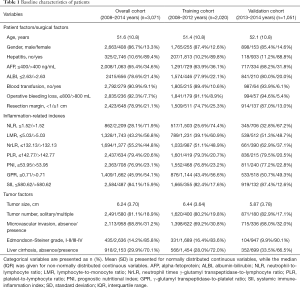
We show the baseline characteristics of patients in Table 1. The included patients had a mean age of 51.6 years (SD, 10.8 years), 86.7% were male, and 89.4% were positive for hepatitis. Pathological examination showed that more than half of the patients had cirrhosis surrounding the hepatic tumor, and 78.6% had albumin-bilirubin (ALBI) grade 1 liver function. In terms of surgical factors, a resection margin of less than 10 mm was observed in 2,423 patients (78.9%); 236 (7.7%) patients suffered an operative bleeding loss amount of more than 800 mL, and 279 (9.1%)required a blood transfusion during the perioperative period. Most patients had single tumors, and the average size of intrahepatic tumors was 6.24 cm (SD, 3.70 cm). Histologically, microvascular invasion and Edmondson-Steiner grades III-IV were recorded in 958 (31.2%) and 2,636 (85.8%) patients, respectively. Seven inflammation-related indexes (including NLR, LMR, NrLR, PLR, PNI, GPR, and SII) are also presented in Table 1. Some characteristics, such as AFP, blood transfusion, operative bleeding loss, and tumor size, and most of the inflammation-related indexes differed slightly between the training and validation cohorts.
Full table
Postoperative prognosis
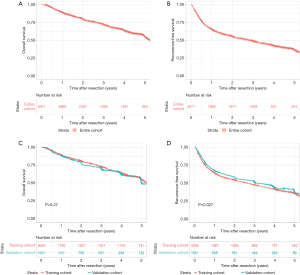
In this study, the median follow-up of all included patients was 46.9 months. Over the 5 years after surgery, 661 people were lost to follow-up, and 801 HCC-related deaths, 254 liver-related deaths, and 138 deaths related to other diseases occurred. The postoperative 1-, 3-, and 5-year OS rates were 88.8%, 71.8%, and 54.7%, respectively, and the 1-, 3-, and 5-year RFS rates were 65.0%, 48.6%, and 36.3%, respectively (Figure S2). The median follow-up was 50.2 and 36.6 months in the training and validation cohorts, respectively. In the training cohort, the postoperative 1-, 3-, and 5-year OS rates were 89.2%, 72.8%, and 55.8%, respectively, and the 1-, 3-, and 5-year RFS rates were 63.9%, 47.2%, and 35.6%, respectively. Meanwhile, the postoperative 1-, 3-, and 5-year OS rates in the validation cohort were 88.2%, 70.0%, and 55.3%, respectively, and the 1-, 3-, and 5-year RFS rates were 67.1%, 51.2%, and 36.8%, respectively. Kaplan-Meier analysis revealed no difference in OS (P=0.27) but a significant difference in RFS (P=0.027) between two cohorts (Figure S2).
Prognostic nomograms for OS and RFS
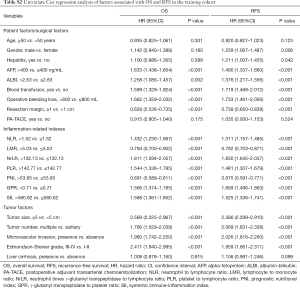
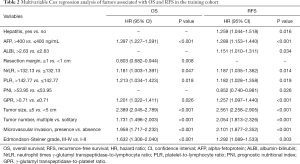
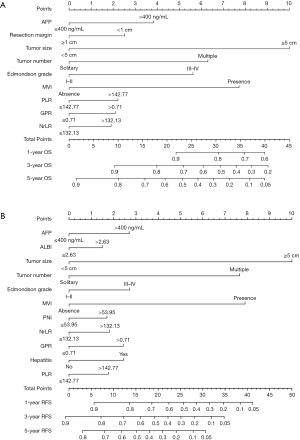
Univariate Cox regression analysis was performed to determine the risk factors associated with OS and RFS in the training cohort. The results are shown in Table S2. Multivariate Cox regression analysis revealed that AFP of >400 ng/mL, a resection margin of <1 cm, NrLR of >132.13, PLR of >142.77, GPR of >0.71, a tumor size of ≥5 cm, multiple tumors, microvascular invasion, and an Edmondson-Steiner grade of III-IV were the independent risk factors associated with OS (Table 2). These factors were incorporated to construct a prognostic nomogram to predict one-, three- and five-year survival (Figure 1A). Based on the nomogram, a simple, easy-to-use predictive tool was developed and is available at https://recurrenceprediction.shinyapps.io/dynnomos/ (Figure S3A).
Full table
Full table
Hepatitis, AFP of >400 ng/mL, ALBI of >2.63, NrLR of >132.13, PLR of >142.77, PNI of ≤53.95, GPR of >0.71, a tumor size of ≥5 cm, multiple tumors, microvascular invasion, and an Edmondson-Steiner grade of III-IV were the independent risk factors associated with RFS by multivariate analysis (Table 2). These variables were used to draw a prognostic nomogram to predict one-, three- and five-year RFS (Figure 1B). Based on the nomogram, a simple predictive tool was developed for clinicians and is available at https://recurrenceprediction.shinyapps.io/dynnomrfs/ (Figure S3B).
Assessing and comparing model performance
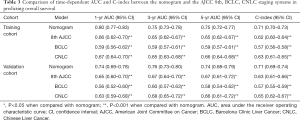
The discriminative performance of the models was assessed and compared by C-index and time-dependent AUC. In predicting OS, the C-index of the nomogram in the training and validation cohorts was 0.71 (95% CI: 0.70–0.73) and 0.71 (95% CI: 0.69–0.74), respectively, which were greater than those of the 8th AJCC staging system (0.62, 95% CI: 0.60–0.64, P<0.001 in the training cohort; 0.63, 95% CI: 0.61–0.66, P<0.001 in the validation cohort), the BCLC staging system (0.57, 95% CI: 0.56–0.58, P<0.001 in the training cohort; 0.57, 95% CI: 0.55–0.59, P<0.001 in the validation cohort), and the CNLC staging system (0.63, 95% CI: 0.61–0.65, P<0.001 in the training cohort; 0.65, 95% CI: 0.62–0.67, P<0.001 in the validation cohort) (Table 3).
Full table
In predicting RFS, the C-index of the nomogram (0.71, 95% CI: 0.70–0.73 in the training cohort; 0.74, 95% CI: 0.72–0.76 in the validation cohort) was also higher than the 8th AJCC, BCLC, and CNLC staging systems (all P<0.001 when compared with the nomogram) (Table 4). Time-dependent AUC (1, 3, and 5 years) showed that the prognostic nomograms for OS and RFS were both significantly greater than the three commonly used staging systems in the training and validation cohorts (Figure 2; Tables 3,4).
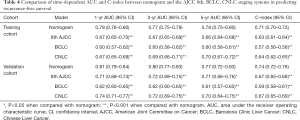
Full table
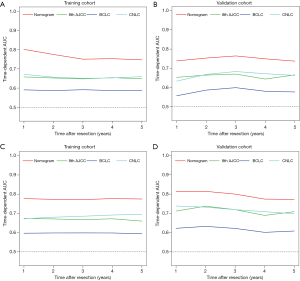
DCA was used to compare clinical usefulness. As shown in Figure 3, DCA was used to calculate the clinical usefulness of each model according to the risk probability threshold (x-axis) and net benefit (y-axis). DCA revealed that the prognostic nomograms had better net benefits in both the training and validation cohorts than the 8th AJCC, BCLC, and CNLC staging systems.
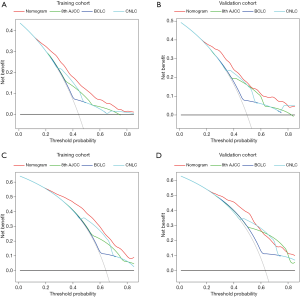
Overall, the calibration curves displayed a good agreement between the prediction of the prognostic nomograms and actual outcomes in the training and validation cohorts (Figure 4).
Subgroup analysis
We validated the current nomograms in different subgroups of patients according to age (≤50 and >50), gender (male and female), etiology (non-viral hepatitis and viral hepatitis), and liver cirrhosis (non-liver cirrhosis and liver cirrhosis). The C-indexes of the two nomograms were still superior to those of the conventional staging systems, suggesting a consistent performance in these populations (Tables S3,S4).

Full table

Full table
Risk stratification
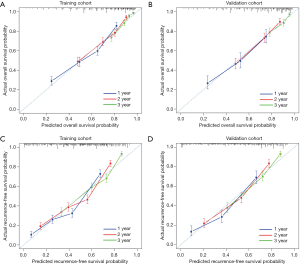
Based on the score calculated by the nomogram for OS, using 20.36 and 29.87 as the cutoff values (which correspond to the 50th and 85th percentile of the score in the training cohort, respectively), the patients were classified into low-risk, intermediate-risk, and high-risk groups. Kaplan-Meier analysis showed that the OS rates stratified prognosis among the three risk groups in the training and validation cohorts (P<0.0001) (Figure S4A,B).
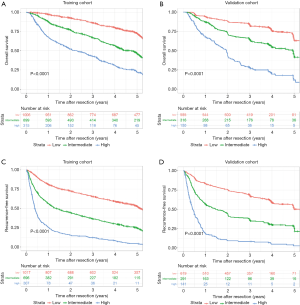
Based on the score calculated by the nomogram for RFS, using 19.58 and 29.03 as the cutoff values (which correspond to the 50th and 85th percentiles of the score in the training cohort, respectively), three distinct risk groups were also stratified. Kaplan-Meier analysis showed that the RFS rates were significantly different among the three risk groups in the training and validation cohorts (P<0.0001) (Figure S4C,D).
Discussion
Based on our large cohort study, we developed and validated novel nomograms to predict prognosis in HCC patients who underwent radical resection. The C-index, time-dependent AUC, and DCA of the nomograms showed significantly better predictive performance than commonly used staging systems. The calibration curves also showed a good correlation between the prediction and the actual outcome. The models could stratify patients into three different risk groups: the low-risk, intermediate-risk, and high-risk groups. These predictive models could facilitate the easy planning of individualized surveillance of surgical patients and postoperative adjuvant therapy trials for high-risk patients to be designed.
Tumor size and number are important stratification criteria in the BCLC staging system (6). Microvascular invasion is also an essential component of the 8th AJCC staging system (5). However, when used alone, these factors are not able to sufficiently reflect the overall malignant characteristics of HCC. Moreover, both the BCLC and 8th AJCC staging systems are not specifically designed to predict HCC recurrence. Given the lack of consensus on risk stratification, present nomograms that combine tumor burden and inflammation-related indexes are more powerful in predicting OS and RFS than the BCLC and 8th AJCC staging systems.
HCC is an inflammatory disease, and there is growing evidence that emphasizes the important role of inflammation in cancer progression (8). Consistent with the results of previous studies, we found NrLR, PLR, and GPR to be independent risk factors of prognosis in HCC (11,12,14). These inflammation-related indexes comprise serum neutrophilia count, lymphopenia count, platelet count, and GGT level. Recent studies have indicated that neutrophils can promote cancer cell proliferation and metastasis through the release of angiogenic factors and inflammatory mediators (25,26). Lymphocytes, on the other hand, play an anticancer role in host immunity by inducing apoptosis and inhibiting cancer cell migration and invasion (8,27). The low platelet count noted in a considerable proportion of patients with cirrhosis is a well-known indicator of portal hypertension (28). Moreover, a high level of GGT was found to be associated with larger tumor size, multiple tumors, and vascular invasion, and was significantly correlated with prognostic outcome in patients with HCC (29,30). However, the molecular mechanism of NrLR, PLR, and GPR in HCC prognosis is still unclear.
We also found that PNI and ALBI were independent risk factors of tumor recurrence in HCC. Chan et al. reported that PNI was a predictor of tumor recurrence in the BCLC 0/A stage HCC after surgical resection (13). ALBI is another independent prognostic indicator of tumor recurrence and is used in SS-CLIP and ERASL models (31,32). Albumin is an important component in PNI and ALBI. Hypoalbuminemia in patients with HCC not only contributes to impaired liver function, caused by the underlying chronic liver disease, but is also associated with a sustained systemic inflammatory response, either from the tumor itself or as a host reaction (33).
There are several limitations to our study. Firstly, the nomograms were established using the data mostly from HBV-infected HCC patients. Markers of chronic liver inflammation, fibrosis, and cirrhosis, including HBV-DNA, HBsAg, and HBeAg, and anti-viral therapy, were not analyzed in this study. Therefore, our results need external validation in different geographic regions and etiology. Secondly, blood cell and liver function levels are affected by infection and metabolic syndrome. Although the results may have incurred some level of bias, infection, and metabolic syndrome can also impact the prognosis of HCC. Thirdly, the application of the nomograms in clinical practice could prove challenging, but simple online tools help to overcome this problem.
In conclusion, two novel nomograms incorporating inflammation-related indexes and accessible clinical parameters were developed to predict OS and RFS in HCC patients who underwent radical resection with adequate performance.
Acknowledgments
Funding: This study was supported by the Special Fund of Fujian Development and Reform Commission (31010308) and the Natural Science Foundation of Fujian Province (2018J01140).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1919
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1919
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1919
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1919). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (No. EHBHKY-2019-11-009). We obtained informed consent from each patient for their data to be used for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 2015;27:933-40. [Crossref] [PubMed]
- Dhir M, Melin AA, Douaiher J, et al. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg 2016;263:1112-25. [Crossref] [PubMed]
- Poon R, Sheung T, Chung M, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-82. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Hanahan D, Weinberg R. Hallmarks of Cancer: The Next Generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 2013;258:301-5. [Crossref] [PubMed]
- Lin ZX, Ruan DY, Li Y, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol 2015;21:10898-906. [Crossref] [PubMed]
- Li J, Liao Y, Suo L, et al. A novel prognostic index-neutrophil times gamma-glutamyl transpeptidase to lymphocyte ratio (NgammaLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep 2017;7:9229. [Crossref] [PubMed]
- Ma W, Zhang P, Qi J, et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Sci Rep 2016;6:35378. [Crossref] [PubMed]
- Chan AWH, Chan SL, Wong GLH, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol 2015;22:4138-48. [Crossref] [PubMed]
- Wang Y, Sun K, Shen J, et al. Novel Prognostic Nomograms Based on Inflammation-Related Markers for Patients with Hepatocellular Carcinoma Underwent Hepatectomy. Cancer Res Treat 2019;51:1464-78. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol 2012;57:1013-20. [Crossref] [PubMed]
- Huang J, Xu L, Luo Y, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol 2014;31:883. [Crossref] [PubMed]
- Chen J, Fang A, Chen M, et al. A novel inflammation-based nomogram system to predict survival of patients with hepatocellular carcinoma. Cancer Med 2018;7:5027-35. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55-63. [Crossref] [PubMed]
- Wang K, Liu J, Yan ZL, et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology 2010;52:164-73. [Crossref] [PubMed]
- Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 2013;13:33. [Crossref] [PubMed]
- Alba AC, Agoritsas T, Walsh M, et al. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA 2017;318:1377-84. [Crossref] [PubMed]
- Vickers AJ, Elkin EB. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med Decis Making 2006;26:565-74. [Crossref] [PubMed]
- Zhou J, Sun HC, Wang Z, et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- Liang J, Piao Y, Holmes L, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res 2014;20:187-98. [Crossref] [PubMed]
- van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347-60. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- de Franchis R, Baveno VF. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. [Crossref] [PubMed]
- Ma H, Zhang L, Tang B, et al. gamma-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol 2014;21:3084-9. [Crossref] [PubMed]
- Fu SJ, Zhao Q, Ji F, et al. Elevated Preoperative Serum Gamma-glutamyltranspeptidase Predicts Poor Prognosis for Hepatocellular Carcinoma after Liver Transplantation. Sci Rep 2016;6:28835. [Crossref] [PubMed]
- Huang S, Huang GQ, Zhu GQ, et al. Establishment and Validation of SSCLIP Scoring System to Estimate Survival in Hepatocellular Carcinoma Patients Who Received Curative Liver Resection. PLoS One 2015;10:e0129000. [Crossref] [PubMed]
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284-93. [Crossref] [PubMed]
- Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 2005;20:369-76. [Crossref] [PubMed]