
Inhaled antibiotics during mechanical ventilation—why it will work
Background—why we want to know if it works
Inhaled antibiotics are commonly used in patients undergoing recurrent or chronic pulmonary infections such as patients suffering from cystic fibrosis (1). Their use is much less frequent among patients under invasive mechanical ventilation suffering of acute infection. Ventilator associated pneumonia (VAP) is a common and severe nosocomial infection among critically ill patient, with a mortality rate of up to 70% (2). American guidelines suggest that inhaled antibiotics remain a last resort treatment for patients not responding to intravenous therapy (3). This recommendation is an expert opinion with a low grade of evidence. In contrast, recent European recommendations do not mention inhaled antibiotics in the setting of VAP and an ESCMID panel positioned against their use putting forward the weak evidence in favor of efficacy and potentially underestimated risks of adverse events (2). However, their use in current practice seems relatively frequent (4).
Delivering antibiotics by intravenous infusion for pneumonia when one can directly target the lungs by aerosolizing may seem curious. As nebulization allows rapid and high concentration of antibiotic delivery to the lungs (5), this route of administration should be preferred on a theoretical point of view. Critically ill patients under mechanical ventilation present modified pharmacokinetics of intravenous antibiotics (6) which further worsen lung tissue drug penetration (7). Increasing the intravenous dose may be an option, which is however limited by toxicity. The use of alternative routes of administration such as nebulization appears a more appealing option, enabling to achieve increased local concentrations with low or inexistent systemic toxicity (8). Moreover, antibiotic resistance is a main side effect of antibiotics and a public health issue identified by World Health Organization (https://www.who.int/fr/news-room/fact-sheets/detail/antibiotic-resistance).
Delivering antibiotics only at the site of infection is one way to avoid resistance emergence, as commensal gut bacteria may be spared and lung antibiotic concentrations are very high. As we will develop it further in this review, two recent trials of inhaled antibiotics were negative despite favorable preclinical data (9,10). We will try to explain these results and focus on how to improve inhaled antibiotic therapy in order to design positive trials in the future.
Advantages—why it should work
Inhaled antibiotics have several theoretical advantages: delivery of high concentration of the antibiotic directly to the targeted infected site (i.e., the lungs); achieving equal or superior therapeutic effect with a fraction of the systemic dose; reducing the risks of side effects compared to the systemic route (5); noninvasive delivery of the therapeutic; ambulatory treatment for chronic ventilated patients through a tracheostomy.
Preclinical data and animal experimentations have appealing results. Goldstein compared inhaled and intravenous amikacin in piglet with E. Coli induced pneumonia: lung tissue concentration was 3- to 30-fold higher after nebulization than after intravenous administration and this was associated with greater bacterial killing. Even in case of severe bronchopneumonia, lung tissue concentrations remained higher with nebulization than after intravenous infusion despite severe pulmonary consolidation (11). This favorable pharmacokinetic profile was confirmed in human studies: among 28 patients with VAP, amikacin aerosolization achieved high concentrations in epithelial lining fluid collected by bronchoalveolar lavage (BAL) and tracheal secretions, constantly above minimum inhibitory concentrations (MIC) of common gram-negative bacteria causing VAP (12).
In 2015, Valachis et al. (13) performed a meta-analysis including 16 studies which compared adjunctive inhaled Colistin to intravenous route alone. There was a significant improvement in clinical response, bacteria eradication and infection-related mortality. There was no effect on overall mortality and nephrotoxicity which is the main concern when administering Colistin. Liu et al. observed similar results (14). In contrast, Zampieri et al. meta-analysis did not observe any benefit of nebulized antibiotic during VAP (15). However, this meta-analysis mixed up several kinds of antibiotics and nebulizers thus potentially increasing the risk of bias due to study heterogeneity.
Constraints—why it has not always worked
Although nebulization is an appealing way for delivering a treatment to the lungs, lots of pitfalls have to be avoided, in particular related to mechanical ventilation when one aims to treat VAP. Indeed, not taking into account the constraints of aerosolization may lead to inefficient therapy, due to insufficient lung deposition. One should keep in mind that the amount of antibiotic loaded in the nebulizer is not the amount of drug deposited in the lung (16). The residual volume remaining in the nebulizer chamber at the end of delivery, extra-pulmonary deposition and aerosol exhalation influence the dose finally deposited in the lung. To optimize nebulization, several parameters have to be taken into account.
The nebulizer used and its position are the first influencing factors. Three types of nebulizers are available: jet nebulizer, ultrasonic nebulizer and vibrating mesh nebulizer. Each has advantages and drawbacks summarized in Table 1. The residual volume and the particle size produced by a given nebulizer are key elements influencing efficiency. Ultrasonic and mesh nebulizers achieved faster and greater lung deposition than jet nebulizer, mainly because of a reduced residual volume (17,18). Furthermore, unlike jet-nebulizer, they do not interfere with the ventilator. Positioning the nebulizer in the inspiratory limb 15 to 40 cm before the Y-piece increases drug delivery (Figure 1). Deposition also depends on bias flow as it induces aerosol loss in the expiratory limb during expiration (18). Mesh nebulizers are to be preferred given their ease of use and efficiency (18). Indeed, the development of dedicated nebulizers designed to be used during mechanical ventilation with dedicated circuits is a key element for successful trials.

Full table
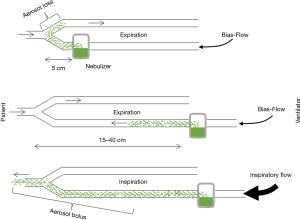
Particle size is an essential factor influencing drug deposition. The aerosolization process produces droplets of variable sizes summarized by the mass median aerosol diameter (MMAD) of the aerosol, i.e., the diameter where 50% of the aerosol mass is made of larger droplets and 50% of the mass is made of smaller particles. Particle size essentially rules deposition, with ventilator circuit impaction being predominant for large droplets >5 µm, bronchial deposition for 3–5 µm droplets, alveolar deposition for 1–3 µm droplets (20,21). Droplets that don’t deposit are exhaled. Thus, particle size distribution has to be taken into account to adapt the nebulizer load according to the expected percentage of antibiotic deposition in the lung. Usually, MMAD between 1 and 5 µm is expected, depending on the targeted site (larger particles may deposit in the proximal airways and treat tracheobronchitis whereas smaller particle size is critical for targeting the alveoli). After deposition, the fate of the drug is variable: removal by mucociliary clearance, degradation and absorption (21).
During invasive mechanical ventilation, inhaled gas needs to be humidified and heated. This contributes to a rise in particle size and consequently modifies aerosol deposition (22). Heat and moisture exchanger represent a barrier to the aerosol and should be removed if they are placed between the patient and the nebulizer. Turning off an active heated humidifier may appear useful to enhance drug delivery but the benefit is questionable as the decrease in heat and humidity is slow (23) and one may forget to turn it back on after nebulization. Dry circuits dedicated to nebulization may be used to enhance nebulization output through immediate change in humidity conditions. In any case, prolonged lack of inspired gas humidification during nebulization (over an hour) induces a risk of tracheal tube occlusion, a very severe complication to be avoided by all means (24).
When the nebulizer residual volume is important, one has to increase the nebulizer drug loading either increasing the concentration of the drug or increasing the volume of the solution loaded in the nebulizer reservoir. In the first case, particle size and thus deposition may be affected; in the second case, duration of nebulization is prolonged which may lead to drug stability concerns (25).
All these considerations made, the last parameter to look after is ventilator settings. Indeed, laminar flow is required to ensure optimal transport of inhaled drugs, which may be compromised by inspiratory efforts of the patient for example. Ideal ventilator settings to enhance aerosol deposition are controlled ventilation with perfect ventilator-patient synchrony, low inspiratory flow (30 L/min), laminar flow patterns and prolonged inspiratory time (26). These settings may be poorly tolerated by awake patients in most of the cases. Benefit of a short sedation during nebulization remains to be evaluated.
Successful antibiotic nebulization requires to optimally implement those various parameters. This said, bringing together all these conditions and achieve a well-managed nebulization is relatively simple. For patients under mechanical ventilation and sedation, it implies to reduce the inspiratory flow, increase inspiratory time, position correctly the nebulizer and remove the heat and moisture exchanging filter. These parameters are not fully taken into account in most of the negative human studies exploring the efficiency of inhaled antibiotics. For example, in the IASIS trial, the nebulizer was placed proximal to the Y piece, humidification was maintained and ventilator setting left unchanged (10). However, simplifying the nebulization setup improves trial feasibility and the optimal tradeoff is difficult to achieve. Development of innovative devices such as the Pulmonary Drug Delivery System combining vibrating mesh nebulization with breath activation may provide clinicians with an effective and very easy to use device. The INHALE study conducted in 153 intensive care units in 25 countries used this innovative device, but showed no difference in survival between the inhaled amikacin and placebo groups [respectively 191 (75%) and 196 (77%) survivors] among patients with resistant Gram negative VAP (9). Adverse events were also similar. Of note, ventilator settings were unmodified during nebulization. Clinical response was achieved with standard of care intravenous antibiotics in 79% of patients infected with a multidrug resistant strain and 75% with extensively drug-resistant strain in the placebo group. This high rate of success in the standard of care group probably contributed heavily to the absence of significant difference between groups. Patients infected with resistant bacteria requiring high dose intravenous antibiotics and exposed to systemic effects are probably the most likely to benefit of inhaled antibiotics; large randomized trials in general recruit much further than this small niche of patients most likely to benefit. The amikacin dose may also be discussed: as a concentration dependent antibiotic, administration of twice a day low dose may have led to the variable alveolar concentrations observed in BAL (27). Among the few patients who underwent a BAL in the INHALE trial, amikacin concentrations were very heterogeneous and very low in one patient. Nevertheless, despite high inter-patient variability, previous results, using the same nebulization setup showed high pulmonary concentration of Amikacin, constantly over the MIC of P. aeruginosa (12). Of note, high concentrations of a nebulized antibiotic in BAL might not always reflect the alveolar concentration, as contamination of the fiberscope may occur by passing through the bronchial tree, where the deposition of nebulized drug is important. Elman et al. (28) observed on infected piglets that Amikacin pulmonary concentration was lower when parenchymal infection was extensive. Thus, tracheal aspirations and/or BAL may not always be representative of deposited antibiotics in very consolidated lung segments (29).
Interestingly, those large negative trials with important feasibility constraints followed several encouraging monocentric positive trials. Lu et al. (8,24) assessed efficacy of inhaled Ceftazidime plus Amikacin in VAP caused by P. aeruginosa, as well as inhaled Colistin in VAP caused by P. aeruginosa and A. baumannii: in both studies, all nebulization optimization techniques were implemented. In case of patient-ventilator asynchrony, patients were sedated. Sterilization of BAL was obtained within 3 days with inhaled Ceftazidime plus Amikacin, including patient infected with P. aeruginosa strains intermediate or resistant to the nebulized antibiotics. Results were consistent with previous preclinical observations in piglets (30). Interestingly, recurrence with resistant bacterial strains occurred only in the intravenous group. Palmer et al. observed concordant results in 24 patients with VAP and/or tracheobronchitis, where no antibiotic resistance appearance was observed after aerosolized antibiotics (31). Similarly, Abdellatif et al. observed that inhaled Colistin was non-inferior to intravenous Colistin with a lower nephrotoxicity in patients suffering VAP (32). Again, ventilator settings were optimized to enhance lung deposition. With the same dose of nebulized Amikacin than the INHALE study, Hassan et al. observed an improved outcomes when co-administered with intravenous Piperacillin/Tazobactam in a monocentric study (33).
Negative results concerning adjuvant Colistin in gram-negative VAP were observed by Rattanaumpawan et al. (34). Ventilator settings and condition of nebulization were not recorded in this study.
Taken together discrepancies between positive and negative trials may in part be related to various implementations of nebulization optimization due to trial feasibility constraints thus inducing various antibiotic concentrations to be expected in the lung and various target patient population more or less likely to benefit of inhaled antibiotics.
Resistance emergence—why it has to work
Multi-drug resistant bacteria (MDR) are increasingly prevalent in critically ill patients and represent a growing concern (35). Curing VAP caused by MDR can be challenging according to the expected optimal pulmonary concentration of intravenous antibiotic, which remains controversial (36,37). Delivering antibiotics by nebulization enables to achieve higher local concentration with no or few systemic effect (11). The differences between inhaled and intravenous antibiotics are summarized in Table 2. It is easy to imagine concentration-dependent antibiotic like Colistin or aminoglycosides to be administered by nebulization; it is way more difficult for time-dependent antibiotic like glycopeptides or ß-lactams, as continuous inhalation leads to specific constraints. However, Morais et al. (38) observed in piglets that a single nebulization of Vancomycin induced higher lung concentration than a single injection. In a prospective observational study of 2,808 critically ill patients, only 1% of nebulization concerned antibiotics. Their use was most of the time related to the treatment of VAP or tracheobronchitis/colonization by MDR bacteria (39). Colistin and aminoglycosides are the most frequently aerosolized antibiotics in intensive care units (40). Thus, current practice illustrates that nebulized antibiotics meet a real need in clinical practice in the setting of spreading MDR bacteria.

Full table
Beginning empirical inhaled antibiotic therapy in case of VAP suspicion, awaiting bacterial identification and antibiotic susceptibility testing to pursue inhaled, intravenous or a combination therapy would be an interesting option to evaluate. Its impact on resistance emergence may be low, when empirical treatment with carbapenems or Piperacillin-Tazobactam is a major cause of resistant strains emergence (35).
Two studies showed no pneumonia recurrence with resistant bacteria when VAP was treated by inhaled antibiotic (24,30). In a comparative phase II trial, Lu et al. (24) compared inhaled vs. intravenous Ceftazidime and Amikacin in 40 patients with VAP caused by P. Aeruginosa. At day 8, success of treatment was similar in both groups with a non-significant favorable effect of inhaled antibiotics (70% success vs. 55%, P=0.33). When pneumonia persisted despite antibiotic treatment, the isolated strains on day 9 remained all susceptible to Ceftazidime and Amikacin in the inhaled group (5/5, 100%) but half were intermediate or resistant in the intravenous group (3/6, 50%). In a double-blind placebo controlled study, Palmer et al. (31) observed similar results: association of inhaled antibiotics to standard of care successfully eradicated MDR bacteria. New drug resistance was seen in 2/16 (13%) patients treated with aerosolized antibiotic vs. 6/11 (55%) in placebo group, P=0.03.
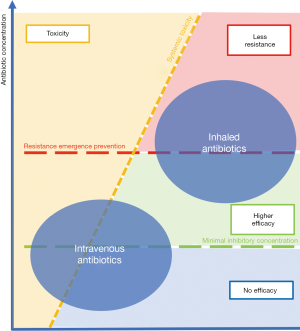
The advantages of inhaled antibiotics compared to intravenous antibiotics concerning toxicity and resistance emergence are represented in Figure 2.
Preventive inhaled antibiotic—it already works
Inhaled antibiotics may be used to prevent VAP. Six comparative trials involving a total of 1,158 patients were included in a meta-analysis in 2018. Prophylactic nebulized antibiotics reduced the occurrence of VAP but had no significant effect on intensive care unit mortality. This prophylactic antibiotic therapy did not increase the occurrence of VAP due to MDR bacteria (41). Antibiotics used in these studies were Colisitin, Ceftazidime and Gentamicin. Another ongoing large scale multicenter double-blind randomized controlled trial will provide further information about prophylactic inhaled Amikacin (NCT03149640).
How to make it work safely
The most common reported adverse events of nebulized antibiotics are tachycardia, hyper- or hypotension, hypoxemia and cough (39). Bronchospasm, usually thought to be a common side effect of nebulization, was unfrequently reported in this large observational study among critically ill patients (39). Obstruction of the ventilator circuit or filters can lead to infrequent but serious outcomes such as pneumothorax or even cardiac arrest (24). Nonetheless, careful monitoring is required during nebulization, especially if the process is meant to be prolonged and/or involves interruption of inhaled gas humidification.
Another safety concern is direct lung toxicity of the inhaled antibiotic as drug formulation may not be designed for this route of administration. Excipients validated for inhaled therapy are listed in the Generally Recognized as Safe (GRAS) list and are limited (https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database). Antibiotics specially formulated to be nebulized should preferably be used. When it is not feasible to use drug formulations specifically developed for inhalation, one may nebulize drugs formulated for intravenous use, ideally free of any known allergenic excipient. For example, in six healthy volunteers, the inhalation of sulfite-free amikacin formulated for intravenous infusion was very well tolerated (5).
Development of new technologies—why it will be easier to make it work
Aerosolization is a wide open field for new technology development. Results from previous studies have to be read with caution as they sometimes used old generation nebulizers. The development of a new generation of nebulizers enhanced lung drug deposition and facilitated routine use. The performance of these new nebulizers has to be validated in clinical trials with adjusted ventilator settings and standardized use.
The INHALE study mentioned above used breath synchronized nebulizers that deliver a bolus of aerosol during a portion of inspiration to reduce expiratory loss of the drug (9). The Pulmonary Drug Delivery System used during this study was a drug-device product designed to achieve high amikacin concentrations in the lungs (12). The device is a pioneering approach of nebulization which enables lower expiratory loss of the nebulized drug (at the cost of prolonged nebulization). However, the negative results of the INHALE study draw attention to the complex pathway of implementing technological advances into the clinical setting. Whereas drug concentrations were very high in tracheal secretions and BAL fluid in pilot trials, in the large scale phase III trial, the antibiotic concentrations measured in a small subset of patients showed extremely large standard deviations, pointing to a lack of precise dose control. Some patients may have been under the limit of detection.
Heliox is a gas mixture of 80% helium and 20% oxygen that has been shown to improve aerosol delivery efficiency during mechanical ventilation up to 50% (42). Mesh nebulizers have minimal change in particle size and do not dilute or change gas administered by the ventilator. The lung deposition of Ceftazidime increased in ventilated piglets when Heliox was used as carrying gas (43). Clinical data are however lacking.
Intra-tracheal devices are also under investigation (44). In 10 ventilated piglets, Amikacin was administered through intravenous infusion, vibrating mesh nebulization or intra-tracheal spray. It was performed using the Microsprayer® device (Penn-Century, Philadelphia, United States) which was positioned under endoscopic control 2 cm distal of the tip of the tracheal tube. This device enabled 94% delivery of the charged Amikacin in a fraction of time vs. 31% for the mesh nebulizer, with MMADs of respectively 37.4 and 6.3 µm. Lung deposition was however variable between and within the piglets after intra-tracheal spray delivery. Intra-tracheal spray requires further investigation as its potential to reduce drug loss and short administration duration are clear advantages.
Another way to optimize aerosol delivery is to address the drug directly to the target. Multiple ways to achieve such targeting are under development such as nanobodies and antibody-drug conjugates (45). For example, one study managed to drive droplets of superparamagnetic nanobodies with the use of a magnetic field in mice (46). Guiding antibiotics directly to the consolidated region of an infected lung could compensate the lack of aerated parenchyma.
Development of new therapies—another way to make it work
Alternatives to antibiotics are meant to be developed considering the growing emergence of MDR bacteria.
Bacteriophages are ubiquitous and able to kill bacteria during their replication cycle. Several experimental studies reported potential benefits of bacteriophages used to treat pulmonary infections and inhaled phage therapy may be clinically relevant and technically feasible for respiratory tract infections (47).
Monoclonal antibodies (mAb) could be another future alternative or adjunctive therapy to antibiotics. Panobacumab is a mAb targeting Pseudomonas aeruginosa. In mice models of pneumonia, it improved bacterial clearance, reduced lung inflammation and had additive effect in combination with Meropenem even in Meropenem resistant strains (48). There is a wide open field to develop pulmonary delivery of anti-infectious mAb through inhalation (49).
Last, immunomodulation may be an appealing alternative or complement to antibiotic therapy. Again, the inhaled route for delivering immunomodulatory agents is of potential interest. Preclinical data in mouse models of pneumonia showed a strong immune response after intranasal delivery of Flagellin, an immune response stimulator derived from flagellated bacteria acting through the Toll-like receptor 5 activation. Inhaled Flagellin combined with antibiotic therapy leads to a faster bacterial clearance than antibiotics alone in mice. The immune response was followed by reestablishment of steady state conditions, assessed on lung biopsies (50,51). This high immune response followed by a rapid return to homeostasis is appealing to treat quickly and efficiently severe lung infections. A European project currently aims at developing a drug and device to nebulize Flagellin in humans (H2020 FAIR project 847786).
Those numerous innovations may drive towards a future of effective “inhaled anti-infectious therapy” rather than “inhaled antibiotics”.
Conclusion—it works
Inhaled antibiotics will work because we hold all the keys to manage it. More generally, inhaled anti-infectious therapy will work: MDR bacteria put pressure towards the development of efficient therapies, based on antibiotics and/or adjuvant or alternatives to it. Physiology, pharmacokinetics and pharmacodynamics all point towards the benefits of inhaled therapy. The current lack of large positive randomized trials illustrates the complexity of the research path from in vitro and technological innovation to the clinical arena, however the continued improvement in identification of patients most likely to benefit, the development of user friendly devices, new technology and improved understanding of lung biology will overcome the last hurdles towards successful implementation of inhaled anti-infections therapy.
Acknowledgments
The authors acknowledge the University Hospital of Tours, France for receipt of the grant “Année Médaille du CHRU de Tours” for M. Desgrouas Master degree; the French society of Intensive Care, Société de Réanimation de Langue Française (SRLF) for receipt of a grant for M. Desgrouas Master degree; The Centre d’Etude des Pathologies Respiratoires (CEPR) of Tours, France, Inserm U1100.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Dr. James B. Fink and Dr. Zhe Luo) for the series “Medical Aerosol in Acute and Critical Care” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3686). The series “Medical Aerosol in Acute and Critical Care” was commissioned by the editorial office without any funding or sponsorship. SE reports grants, personal fees and non-financial support from Aerogen Ltd, grants, personal fees and non-financial support from Fisher and Paykel healthcare, grants from Hamilton medical, personal fees from La Diffusion Technique Française, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mogayzel PJ, Naureckas ET, Robinson KA, et al. Cystic Fibrosis Pulmonary Guidelines: Chronic Medications for Maintenance of Lung Health. Am J Respir Crit Care Med 2013;187:680-9. [Crossref] [PubMed]
- Rello J, Solé-Lleonart C, Rouby J-J, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect 2017;23:629-39. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-111. [Crossref] [PubMed]
- SANEME-2 Investigators. Nebulization of antimicrobial agents in mechanically ventilated adults in 2017: an international cross-sectional survey. Eur J Clin Microbiol Infect Dis 2018;37:785-94. [Crossref] [PubMed]
- Ehrmann S, Mercier E, Vecellio L, et al. Pharmacokinetics of high-dose nebulized amikacin in mechanically ventilated healthy subjects. Intensive Care Med 2008;34:755-62. [Crossref] [PubMed]
- de Montmollin E, Bouadma L, Gault N, et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 2014;40:998-1005. [Crossref] [PubMed]
- Rodvold KA, George JM, Yoo L. Penetration of Anti-Infective Agents into Pulmonary Epithelial Lining Fluid. Clin Pharmacokinet. 2011;50:637-64. [Crossref] [PubMed]
- Lu Q, Zahr N, Rouby J-J. Efficacy of High-dose Nebulized Colistin in Ventilator- associated Pneumonia Caused by Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012;117:1335-47. [Crossref] [PubMed]
- Niederman MS, Alder J, Bassetti M, et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect Dis 2020;20:330-40. [Crossref] [PubMed]
- Kollef MH, Ricard J-D, Roux D, et al. A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia. Chest 2017;151:1239-46. [Crossref] [PubMed]
- Goldstein I, Wallet F, Nicolas-Robin A, et al. Lung Deposition and Efficiency of Nebulized Amikacin during Escherichia coli Pneumonia in Ventilated Piglets. Am J Respir Crit Care Med 2002;166:1375-81. [Crossref] [PubMed]
- Luyt C-E, Clavel M, Guntupalli K, et al. Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care 2009;13:R200. [Crossref] [PubMed]
- Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med 2015;43:527-33. [Crossref] [PubMed]
- Liu D, Zhang J, Liu H-X, et al. Intravenous combined with aerosolised polymyxin versus intravenous polymyxin alone in the treatment of pneumonia caused by multidrug-resistant pathogens: a systematic review and meta-analysis. Int J Antimicrob Agents 2015;46:603-9. [Crossref] [PubMed]
- Zampieri FG, Nassar AP Jr, Gusmao-Flores D, et al. Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care 2015;19:150. [Crossref] [PubMed]
- Dugernier J, Ehrmann S, Sottiaux T, et al. Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit Care 2017;21:264. [Crossref] [PubMed]
- Harvey CJ, O’Doherty MJ, Page CJ, et al. Comparison of jet and ultrasonic nebulizer pulmonary aerosol deposition during mechanical ventilation. Eur Respir J 1997;10:905-9. [PubMed]
- Ari A, Atalay OT, Harwood R, et al. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care 2010;55:845-51. [PubMed]
- Ehrmann S, Chastre J, Diot P, et al. Nebulized antibiotics in mechanically ventilated patients: a challenge for translational research from technology to clinical care. Ann Intensive Care 2017;7:78. [Crossref] [PubMed]
- Köbrich R, Rudolf G, Stahlhofen W. A Mathematical Model of Mass Deposition in Man. Ann Occup Hyg 1994;38:15-23.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003;56:588-99. [Crossref] [PubMed]
- Miller DD, Amin MM, Palmer LB, et al. Aerosol Delivery and Modern Mechanical Ventilation: In Vitro/In Vivo Evaluation. Am J Respir Crit Care Med 2003;168:1205-9. [Crossref] [PubMed]
- Lin HL, Fink JB, Zhou Y, et al. Influence of Moisture Accumulation in Inline Spacer on Delivery of Aerosol Using Metered-Dose Inhaler During Mechanical Ventilation. Respir Care 2009;54:1336-41. [PubMed]
- Lu Q, Yang J, Liu Z, et al. Nebulized Ceftazidime and Amikacin in Ventilator-associated Pneumonia Caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med 2011;184:106-15. [Crossref] [PubMed]
- Wallace SJ, Li J, Rayner Craig R, et al. Stability of Colistin Methanesulfonate in Pharmaceutical Products and Solutions for Administration to Patients. Antimicrob Agents Chemother 2008;52:3047-51. [Crossref] [PubMed]
- Ehrmann S, Luyt CE. Optimizing aerosol delivery of antibiotics in ventilated patients. Curr Opin Infect Dis 2020;33:197-204. [Crossref] [PubMed]
- Moore RD, Lietman PS, Smith CR. Clinical Response to Aminoglycoside Therapy: Importance of the Ratio of Peak Concentration to Minimal Inhibitory Concentration. J Infect Dis 1987;155:93-9. [Crossref] [PubMed]
- Elman M, Goldstein I, Marquette C-H, et al. Influence of Lung Aeration on Pulmonary Concentrations of Nebulized and Intravenous Amikacin in Ventilated Piglets with Severe Bronchopneumonia Anesthesiology 2002;97:199-206. [Crossref] [PubMed]
- Rouby JJ, Monsel A, Ehrmann S, et al. The INHALE trial: multiple reasons for a negative result. Lancet Infect Dis 2020;20:778-9. [Crossref] [PubMed]
- Ferrari F, Liu Z-H, Lu Q, et al. Comparison of lung tissue concentrations of nebulized ceftazidime in ventilated piglets: ultrasonic versus vibrating plate nebulizers. Intensive Care Med 2008;34:1718-23. [Crossref] [PubMed]
- Palmer LB, Smaldone GC. Reduction of Bacterial Resistance with Inhaled Antibiotics in the Intensive Care Unit. Am J Respir Crit Care Med 2014;189:1225-33. [Crossref] [PubMed]
- Abdellatif S, Trifi A, Daly F, et al. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care 2016;6:26. [Crossref] [PubMed]
- Hassan NA, Awdallah FF, Abbassi MM, et al. Nebulized Versus IV Amikacin as Adjunctive Antibiotic for Hospital and Ventilator-Acquired Pneumonia Postcardiac Surgeries: A Randomized Controlled Trial*. Crit Care Med 2018;46:45-52. [Crossref] [PubMed]
- Rattanaumpawan P, Lorsutthitham J, Ungprasert P, et al. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother 2010;65:2645-9. [Crossref] [PubMed]
- Barbier F, Pommier C, Essaied W, et al. Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother 2016;71:1088-97. [Crossref] [PubMed]
- Imberti R, Cusato M, Villani P, et al. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010;138:1333-9. [Crossref] [PubMed]
- Markou N, Fousteri M, Markantonis SL, et al. Colistin Penetration in the Alveolar Lining Fluid of Critically Ill Patients Treated With IV Colistimethate Sodium. Chest 2011;139:232-3. [Crossref] [PubMed]
- Morais CLM, Nascimento JWL, Ribeiro AC, et al. Nebulization of Vancomycin Provides Higher Lung Tissue Concentrations than Intravenous Administration in Ventilated Female Piglets with Healthy Lungs. Anesthesiology 2020;132:1516-27. [Crossref] [PubMed]
- Ehrmann S, Roche-Campo F, Bodet-Contentin L, et al. Aerosol therapy in intensive and intermediate care units: prospective observation of 2808 critically ill patients. Intensive Care Med 2016;42:192-201. [Crossref] [PubMed]
- Ehrmann S, Roche-Campo F, Sferrazza Papa GF, et al. Aerosol therapy during mechanical ventilation: an international survey. Intensive Care Med 2013;39:1048-56. [Crossref] [PubMed]
- Póvoa FCC, Cardinal-Fernandez P, Maia IS, et al. Effect of antibiotics administered via the respiratory tract in the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. J Crit Care 2018;43:240-5. [Crossref] [PubMed]
- Goode ML, Fink JB, Dhand R, et al. Improvement in aerosol delivery with helium-oxygen mixtures during mechanical ventilation. Am J Respir Crit Care Med. 2001;163:109-14. [Crossref] [PubMed]
- Tonnellier M, Ferrari F, Goldstein I, et al. Intravenous versus nebulized ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology 2005;102:995-1000. [Crossref] [PubMed]
- Guillon A, Darrouzain F, Heuzé-Vourc’h N, et al. Intra-tracheal amikacin spray delivery in healthy mechanically ventilated piglets. Pulm Pharmacol Ther 2019;57:101807 [Crossref] [PubMed]
- Bodier-Montagutelli E, Mayor A, Vecellio L, et al. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expert Opin Drug Deliv 2018;15:729-36. [Crossref] [PubMed]
- Dames P, Gleich B, Flemmer A, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol 2007;2:495-9. [Crossref] [PubMed]
- Bodier-Montagutelli E, Morello E, L’Hostis G, et al. Inhaled phage therapy: a promising and challenging approach to treat bacterial respiratory infections. Expert Opin Drug Deliv 2017;14:959-72. [Crossref] [PubMed]
- Secher T, Fas S, Fauconnier L, et al. The Anti-Pseudomonas aeruginosa Antibody Panobacumab Is Efficacious on Acute Pneumonia in Neutropenic Mice and Has Additive Effects with Meropenem. PLoS One 2013;8:e73396 [Crossref] [PubMed]
- Desoubeaux G, Reichert JM, Sleeman M, et al. Therapeutic monoclonal antibodies for respiratory diseases: Current challenges and perspectives, March 31 – April 1, 2016, Tours, France. mAbs 2016;8:999-1009. [Crossref] [PubMed]
- Muñoz N, Van Maele L, Marques JM, et al. Mucosal Administration of Flagellin Protects Mice from Streptococcus pneumoniae Lung Infection. Infect Immun 2010;78:4226-33. [Crossref] [PubMed]
- Porte R, Fougeron D, Muñoz-Wolf N, et al. A Toll-Like Receptor 5 Agonist Improves the Efficacy of Antibiotics in Treatment of Primary and Influenza Virus-Associated Pneumococcal Mouse Infections. Antimicrob Agents Chemother 2015;59:6064-72. [Crossref] [PubMed]


