
Medulloblastoma malignant biological behaviors are associated with HOTAIR/miR-483-3p/CDK4 axis
Introduction
Brain tumors are the most common malignancy in children, and among them, medulloblastoma having the highest incidence, about 8 to 10 percent (1). The World Health Organization (WHO) defines medulloblastoma as “a malignant, invasive embryonal tumor of the cerebellum with a preferential manifestation in children, predominantly neuronal differentiation and an inherent tendency to metastasize via cerebrospinal pathways.” (2). Medulloblastoma was first introduced by Harvey Cushing and Percival Bailey, and it tends to occur in children under the age of 10 (3). In the last decades, the treatment for medulloblastoma has significantly improved, including surgery, chemotherapy, and radiation therapy (4). Nevertheless, after poor prognosis, 5-year survival rates of patients with relapsed disease are approximately 25% (5). Thus, more efficient treatment strategies need to be found.
Long noncoding RNAs (lncRNAs) are non-protein coding transcripts of more than 200 nucleotides in length. They play essential roles in the malignant behavior of cancer (6). HOX antisense intergenic RNA (HOTAIR) is one of the best-studied lncRNAs. HOTAIR is located on chromosome 12q13.13, and it is a polyadenylated RNA with 2158 nucleotides and 6 exons (7). It has been proved to be related to the metastasis of various tumors, including breast cancer (8), prostate cancer (9), renal cell carcinoma (10), squamous cell carcinoma (11) and so on. HOTAIR is also highly expressed in medulloblastoma (12). The molecular mechanism of HOTAIR in medulloblastoma needs to be verified.
In earlier studies, an exceptional expression of HOTAIR is closely related to tumor angiogenesis, tumor microenvironment, and tumor immunity. In nasopharyngeal carcinoma, HOTAIR activated the transcription of angiogenic factor vascular endothelial growth factor A (VEGF-A) directly to promote angiogenesis (13). For liver cancer, HOTAIR promotes the proliferation and growth of liver cancer through the down-regulation of miR-217 (14). So HOTAIR may have a targeted relationship with mi-RNAs.
MicroRNAs (miRNAs) are noncoding RNAs that can modulate gene expression. They are about 18–28 ribonucleotide lengths (15). MiR-483-3p is a mature miRNA that studied its function on different cancer-related pathways (16). It has been proved to be abnormally expressed in some cancer cells, including in esophageal squamous cell carcinoma (17) and breast cancer (18). Nevertheless, it is unclear whether miR483-3p is overexpressed or underexpressed in medulloblastoma.
In this study, we explored the mechanism for HOTAIR to regulate the medulloblastoma malignant biological behaviors. We showed the targeting relationships between HOTAIR and miR-483-3p, miR-483-3p, and CDK4. This study may supply a new target for medulloblastoma treatment.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5006).
Methods
Cell lines and cell culture
Human medulloblastoma cell lines Daoy and D341 were bought from the Cell Bank of Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. Cells were maintained in high-glucose Dulbecco’s modified eagle medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 µg/mL streptomycin, and 100 µg/mL penicillin (Hyclone, Logan, UT, USA). Cells were cultured at 37 °C in a humidified 5% CO2 atmosphere. The culture medium was refreshed every 48 hours.
Plasmids and transfection
The sequence of human HOTAIR shRNA was designed with the BLOCK‐iT™ RNAi Designer (Invitrogen). The sequence was chemically synthesized and cloned into the pcDNA3.1 vector. PCR amplified full-length CDK4 using primers to construct the CDK4 expression plasmid. Then CDK4 was cloned into the pcDNA3.1 vector (Invitrogen). The miR-483-3p inhibitor and miR-483-3p mimic were synthesized by GenePharma Company (Shanghai, China). For transfection, cells were seeded and transfected according to the manufacturer’s instructions of Lipofectamine TM2000 (Invitrogen, Carlsbad, CA).
Quantitative real-time PCR (qRT-PCR)
Total RNA from cells was extracted with an RNA extraction kit (Qiagen, Venlo, the Netherlands) according to the manufacturer’s instructions. Total cDNA was synthesized after equal amounts of RNA were reversely transcribed by a one-step PrimeScript miRNA cDNA synthesis kit (Takara, Dalian, China). The RT-PCR was performed as previously described (19). The primers of HOTAIR, miR-483-3p, and CDK4 were synthesized by Sangon Biotech (Shanghai, China). And qPCR was performed using the ABI Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA). The expressions were calculated using 2−ΔΔCt and normalized with Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). HOTAIR-WT, wild-type HOTAIR; HOTAIR-MUT, mutant HOTAIR.
BrdU staining
The amount of BrdU is a factor in determining the ability of cell proliferation. It is measured by the APC-BrdU Flow Kit (Cat Nr. 552598, BD Pharmingen™) according to the manufacturer’s instruction. Eight randomly selected fields were involved in analyze for each slide. The BD FACSDiva software analyzed the results.
Colony formation
Medulloblastoma cells Daoy and D341 transfected with different treatments were placed into 6 cm dishes. Each group was repeated three times. These dishes were incubated at 37 °C for 2 weeks to allow colony formation. The clones were washed twice with PBS, fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet. Stained colonies were photographed with a digital camera and analyzed with Image Pro Plus.
Western blotting
The primary antibodies, including anti-Ki67, anti- proliferating cell nuclear antigen (PCNA), anti-survivin, anti-caspase-3, anti-caspase-9, anti-Bcl-2, anti-Bax, anti-CDK4 were purchased from Abcam (Cambridge, UK). Cells of different treatments were cultured for 48 hours and then lysed with RIPA lysis buffer. A BCA kit (Pierce, Rockford, IL, USA) was used to determine the total protein concentration. An equal amount of protein was isolated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The membranes were blocked by 5% non-fat dry milk and incubated with anti-Ki67, anti-PCNA, anti-survivin, anti-caspase-3, anti-caspase-9, anti-Bcl-2, anti-Bax, anti-CDK4 at 4 °C overnight. Then the membranes were washed by TBST buffer and subjected to the secondary antibody. An ECL detection kit detected the blots (Pierce, Rockford, IL, USA). GAPDH was used as a loading control.
Hoechst 33342 staining
Morphological examination of cells can be observed by Hoechst 33342 staining. Daoy and D341 cells with different treatments were incubated for 48 hours. Then cells were washed with PBS twice and fixed with 4% paraformaldehyde for 10 minutes. The fixed cells were stained with Hoechst 33342 for 5 minutes, and the staining results were observed with a fluorescence microscope.
Flow cytometry
Flow cytometry assays were conducted using the annexin V/PI apoptosis kit according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Daoy and D341 cells with different treatments were seeded in 6-well plates (1×106 cells/well) and cultured for 48 hours. The cells were harvested by trypsinization and washed by cold PBS, then resuspended in 100 µL binding buffer at a density of 106 cells/mL. Annexin V-FITC and propidium iodide (PI) were added to the suspension. The suspension was incubated for 15 min in the dark at room temperature, then binding buffer was added. Finally, flow cytometry analyzed the samples.
Luciferase reporter assay
Wild-type HOTAIR fragments holding possible binding sites of miR-483-3p were amplified from human genomic DNA by PCR. Seed region mutagenesis was achieved by using a mutate reverse primer. Wild-type HOTAIR (HOTAIR-WT) and mutant HOTAIR (HOTAIR-MUT) fragments were inserted into a downstream of the pmirGLO promoter vector luciferase gene to generate recombinant plasmids HOTAIR-WT and HOTAIR-MUT, respectively. In luciferase reporter assay, recombinant plasmids were transiently co-transfected with miR-483-3p mimics and knocked into Daoy cells via LipofectamineTM2000 (Life Technologies, Carlsbad, CA). After being transfected for 24 hours, the dual-luciferase reporter assay system (Promega) was used to assess luciferase activity according to the manufacturer’s instructions.
Xenograft model of medulloblastoma
Twelve athymic Balb/C nude mice were bought from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). All the mice were 6-week-old and about 20 g weight. These mice were divided into two groups, control group and transfected with shRNA-HOTAIR. Untransfected and transfected with shRNA-HOTAIR Daoy cells were subcutaneously injected into nude mice in the control group and shRNA-HOTAIR group, respectively. All the mice were sacrificed after 30 days. All the tumors were dissected and analyzed. ICH were applied with the fixed tumors according to protocol.
Experiments were performed in compliance with local ethics and the Use Committee for Animal Care and performed in accordance with institutional guidelines. The Sub-Committee approved the study on Biomedical Ethics, Affiliated Hospital of North Sichuan Medical College (2018-61).
Immunofluorescence assay
Tissues from the tumor models were placed on coverslips, fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10min, and permeabilized with 0.1% Triton X-100/PBS. Then, the slides were blocked with 1% BSA for 1 h and incubated overnight with primary antibodies against PCNA and Ki67 at 4 °C. After that, slides were incubated with FITC-conjugated secondary antibody (Beyotime) for 1h. The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). The fluorescence imaging was analyzed by a confocal laser scanning microscopy (Carl Zeiss, Oberkochen, Germany).
TUNEL assay
Paraffin sections of tumor tissue were assembled to investigate cell apoptosis in tumor cells. TUNEL was used to analyze apoptosis. The TUNEL was carried out as previously described (20).
Statistical analysis
GraphPad Prism software processed all the data, and the results were expressed as mean ± standard deviation (SD) from at least 3 replicates. Student’s t-test was conducted to compare the differences between all the groups. A significant difference was considered when A P value of less than 0.05.
Results
Down-regulation of HOTAIR inhibited the proliferation in medulloblastoma cells
shRNA, specifically targeting HOTAIR, was transfected into two medulloblastoma cell lines, Daoy and D341. The result of RT-PCR showed that the expression of HOTAIR was significantly down-regulated in shRNA-HOTAIR group compared to the control group (Figure 1A). Then, BrdU-label-retaining and a colony formation assay were performed to examine cell proliferation. The result showed that BrdU-positive cells were markedly induced compared to the control group (Figure 1B,C). Both the number of colonies and colony-forming rates were much lower than the control group (Figure 1D,E). Furthermore, expressions of Ki67, PCNA, and Survivin were detected by Western blotting (Figure 1F). In the shRNA-HOTAIR group, the expressions of Ki67, PCNA, and Survivin were all dramatically inhibited by shRNA compared to the control group (Figure 1G,H,I). These results taken together showed down-regulation of HOTAIR inhibited the proliferation of medulloblastoma cells, Daoy, and D341.

Down-regulation of HOTAIR increased the apoptosis in medulloblastoma cells
Both cells were transfected with shRNA to investigate the effect of shRNA transfection on cell apoptosis of Daoy and D341 cells. As shown in Figure 2A and B, Hoechst 3322 staining showed that the cellular nuclei of shRNA-HOTAIR became fragmented, and the cell apoptosis rate was increased in the shRNA-HOTAIR compared to the control group. Quantitative analysis of cell apoptosis rate also showed that the apoptosis rate increased significantly (Figure 2C,D). Cleaved-caspase-3 and cleaved-caspase-9 are the active forms of caspase-3 and caspase-9, respectively. The result of western blotting showed that the expressions of cleaved-caspase-3, cleaved caspase-9, and Bax was upregulated, whereas the expression of bcl-2 was down-regulated (Figure 2E). Furthermore, we found that the ratios of Bax to bcl-2 (Figure 2F), cleaved-caspase-3 to caspase-3 (Figure 2G), cleaved-caspase-9 to caspase-9 (Figure 2H) were significantly higher in the shRNA-HOTAIR group than in the control group. These results showed that interference of HOTAIR induced cell apoptosis of medulloblastoma cells.
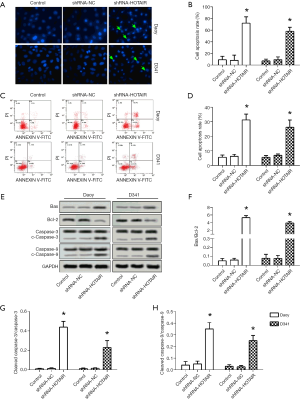
MiR-483-3p was a direct target of HOTAIR in medulloblastoma cells
The TargetScan and miRanda prediction algorithms predicted that there was a targeted relationship between HOTAIR and miR-483-3p (Figure 3A). To determine the targeted relationship between HOTAIR and miR-483-3p, we chose Daoy cells for the later experiments. First, the miR-483-3p inhibitor or mimics were transfected into Daoy cells; the relative level of miR-483-3p was significantly increased with mimics transfection while inhibited in the inhibitor group compared to the control group, as shown in Figure 3B. RT-PCR results showed that the expression of HOTAIR was dramatically higher in shRNA-HOTAIR while lower in miR-483-3p inhibitor group than that in the control group (Figure 3C). Similarly, compared to control, level of miR-483-3p was decreased in shRNA-HOTAIR group (Figure 3D). Furthermore, the targeted relationship between HOTAIR and miR-483-3p was further determined by luciferase activity assay. Compared to cells transfected with HOTAIR-WT, HOTAIR-MUT, and cells co-transfected with HOTAIR-MUT and miR-483-3p mimic, the luciferase activity was significantly decreased in the cells co-transfected with HOTAIR-WT and miR-483-3p mimic (Figure 3E). These results, taken together, suggested a direct targeting relationship of miR-483-3p by HOTAIR.
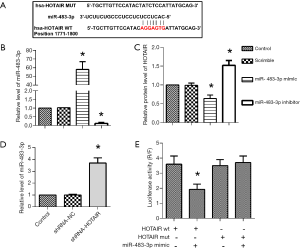
MiR-483-3p directly targeted CDK4 in medulloblastoma cells
The TargetScan and miRanda prediction algorithms showed that there was a binding site between miR-483-3p and CDK4 (Figure 4A). Daoy cells were transfected with pcDNA-CDK4, then RT-PCR analysis confirmed the reduced expression of CDK4 (Figure 4B). Also, CDK4 was reduced with shRNA-HOTAIR (Figure 4C). The targeting of CDK4 with miR-483-3p was validated with luciferase reporter assay. As expected, luciferase activity was markedly reduced in CDK4 wt and miR-483-3p co-transfected cells (Figure 4D). Daoy cells were divided into four groups, control group, and groups transfected with shRNA-HOTAIR, pcDNA-CDK4, shRNA-HOTAIR+CDK4, respectively. The expression of CDK4 was significantly inhibited by shRNA-HOTAIR, while pcDNA-CDK4 promoted it compared to the control group. Also, the expression of CDK4 was suppressed in the shRNA-HOTAIR+CDK4 group compared to the pcDNA-CDK4 group (Figure 4E). The result of the colony formation assay showed shRNA-HOTAIR inhibited cell proliferation, but pcDNA-CDK4 increased the proliferation of Daoy compared to the control group. The shNRA-HOTAIR partially restored the CDK4-induced increase of cell proliferation (Figure 4F,G). The results of flow cytometry indicated that compared to the control group, apoptotic cells increased significantly in Daoy cells transfected with shRNA-HOTAIR while it was decreased in Daoy cells transfected with pcDNA-CDK4 (Figure 4H,I). Quantitative analysis of cell apoptosis rate also showed comparable results. Besides, the cell apoptosis rate in the shRNA-HOTAIR+CDK4 group was higher than in the pcDNA-CDK4 group. In summary, these assays proved miR-483-3p directly targeted CDK4 in medulloblastoma cells.
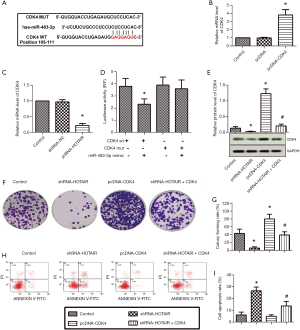
Inhibition of HOTAIR suppressed medulloblastoma tumor growth in a xenograft model
A subcutaneous xenograft model of medulloblastoma in nude mice was built to investigate further whether the down-regulation of HOTAIR could suppress the growth of medulloblastoma tumors in vivo. The nude mice were divided into two groups, one group inoculated with Daoy cells transfected with shRNA-HOTAIR, and the other inoculated with Daoy cells were transfected with shRNA-NC. Assays were done after 30 days. Tumor sizes were visually smaller in mice with shRNA-HOTAIR-transfected Daoy cells than in control mice (Figure 5A). Then mean tumor weight was significantly decreased in the shRNA-HOTAIR group than the control group (Figure 5B). The results of RT-PCR showed that the expressions of HOTAIR and CDK4 were down-regulated while the expression of miR-483-3p was dramatically upregulated by shRNA-HOTAIR (Figure 5C). The results of immunohistochemical staining showed that Ki67 and PCNA in shRNA-HOTAIR groups were decreased compared to control groups (Figure 5D,E,F). The TUNEL assay showed that apoptotic cells were remarkably increased in shRNA-HOTAIR groups (Figure 5D,G). Taken together, these in vivo results showed that inhibition of HOTAIR suppressed tumor growth and promoted apoptosis in medulloblastoma cells, through upregulating miR-483-3p and downregulating of CDK4 (Figure 5H).
Discussion
HOTAIR is one of the best-studied lncRNAs; its effect on tumors has been investigated in many cancers. It is vital to the malignant behavior of cancer (21). Expressions of HOTAIR were aberrantly increased in breast cancer (8), prostate cancer (9), renal cell carcinoma (10), etc. The same situation was found in the pediatric brain tumors including medulloblastoma (12). More impressively, the down-regulation of HOTAIR could inhibit the progression of esophageal cancer (22). Hence, we speculated that down-regulation of the expression of HOTAIR could inhibit cell proliferation in medulloblastoma cells. Ki67 is an intrinsic marker of cell proliferation (23), PCNA is a direct indicator of cell proliferation (24), survivin is an anti-apoptosis protein which can inhibit cell apoptosis (25). The expression of these three proteins can reflect the level of cell proliferation. In this study, when HOTAIR expression was inhibited, all the results showed that cell proliferation was inhibited too.
The relationship between HOTAIR and cell apoptosis has been investigated by many researchers. Works from multiple research teams have shown that regulation of HOTAIR expression levels can induce apoptosis in tumor cells by regulating different signaling pathways (26-28). There are two main pathways of cell apoptosis, extracellular pathway, and intracellular pathway (29). Caspase-3 is a co-executive protein of both pathways, and increased expression of its active form cleaved-caspase-3 suggests increasing cell apoptosis. In the intracellular pathway, cleaved-caspase-3, which is necessary for many apoptotic nuclear changes, comes from procaspase-3 cleaved by active caspase-9 (30). The Bcl-2 family is an essential regulatory pathway of cell apoptosis, and bcl-2 protein is an inhibitor of cell apoptosis, while Bax is exactly the opposite; its overexpression can promote the occurrence of cell apoptosis (31). Caspase-3, caspase-9, and Bax have been proved to be related to pro-apoptosis; Bcl-2 is considered a significant factor of anti-apoptosis. In this study, after inhibition of HOTAIR expression, cell apoptosis rate was decreased, the ratios of Bax/Bcl-2, cleaved-caspase-3/caspase-3, cleaved-caspase-9/caspase-9 were increased. These results showed that HOTAIR inhibited the apoptosis of medulloblastoma cells.
The interaction between lncRNA and miRNA in cancers has been studied. HOTAIR contributes to the proliferation and growth of liver cancer via targeting miR-217 (14), HOTAIR could specifically bind to miR-204 as a competing endogenous RNA in esophageal cancer (22). LncRNA MGE3 mediated its protective effects on hepatocellular carcinoma via binding to miR-483-3p (32). In this study, the potential interaction between HOTAIR and miR-483-3p was proved. Daoy cell transfected with shRNA-HOTAIR, the expression of miR-483-3p, was significantly increased. It showed that down-regulation HOTAIR promoted the expression of miR-483-3p; there was a targeted relationship between HOTAIR and miR-483-3p. This result agreed with bioinformatics prediction and luciferase reporter assays.
CDK4 is a crucial promoter of cell proliferation, and it is overexpressed in many tumors. CDK4 inhibitors have become potential candidates for cancer treatment (33). A selective inhibitor of CDKs 4 and 6, provides a significant therapeutic benefit in medulloblastoma (34). In this study, the down-regulation of HOTAIR induced a decrease of CDK4 and an increase in cell apoptosis in Daoy cell. The results correspond to the earlier studies mentioned above. The cell proliferation of Daoy cell co-transfected with shRNA-HOTAIR and pcDNA-CDK4 was highly decreased compared to Daoy cell transfected with pcDNA-CDK4 while cell apoptosis was on the contrary. According to a previous report, CDK4 is a direct target of miR-483-3p in normal human keratinocytes and human immortalized keratinocytes (35). We showed that CDK4 is a target of miR-483-3p in medulloblastoma cells.
A xenograft model of medulloblastoma was set up with the Dayo cell line. Then data in vivo showed suppression of HOTAIR inhibited the growth of the tumor. In tumor tissues, the expressions of HOTAIR and CDK4 were notably reduced while the expression of miR-483-3p was increased. Cell proliferation-related proteins Ki67 (23) and PCNA (24) were decreased simultaneously. A similar study reported that miR-483-3p significantly hampered tumor growth in subcutaneous squamous cell carcinoma xenografts (36). This evidence, in combination with results in this study, illustrated that down-regulation of HOTAIR suppressed medulloblastoma tumor growth and promoted cell apoptosis in tumor tissues through the up-regulation of miR-483-3p. CDK4 inhibitors were employed in clinical researches on cancer, including esophageal squamous cell carcinoma (37), intrahepatic cholangiocarcinoma (38), pancreatic carcinoma (39). These pieces of research proved that low expression of CDK4 could promote apoptosis in cancer cells. Depending on these results, it was clear that the down-regulation of HOTAIR promotes the apoptosis in tumor tissues via negative regulation of CDK4 by miR-483-3p.
There are limitations in this work. First, the increased miR-483-3p and decreased CDK4 obviously was just part of downstream of HOTAIR, and only the two molecules were investigated. What is more, the regulation and downstream of CDK4 were not studied. All these needs further investigation. Anyway, we have showed a possible way of action for HOTAIR, which could help understanding the role of HOTAIR in medulloblastoma as well as other tumors.
In summary, we found that down-regulation of HOTAIR inhibited cell proliferation and promoted cell apoptosis in medulloblastoma cells by up-regulation of miR-483-3p and down-regulation of CDK4, respectively. All the data above suggest HOTAIR can be used as a candidate target for molecular targeting treatment of human medulloblastoma and the development of anticancer drugs.
Acknowledgments
Funding: This work was supported by the Science and Technology Planning Project of Sichuan (2011JY0062) and Key project of the Affiliated Hospital of North Sichuan Medical College (2020ZD018).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5006
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5006
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5006). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed in compliance with local ethics and the Use Committee for Animal Care and performed in accordance with institutional guidelines. The Sub-Committee approved the study on Biomedical Ethics, Affiliated Hospital of North Sichuan Medical College (2018-61).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Archer TC, Mahoney EL, Pomeroy SL. Medulloblastoma: Molecular Classification-Based Personal Therapeutics. Neurotherapeutics 2017;14:265-73. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Sun Y, Liu G, Chen W, et al. Dosimetric comparisons of craniospinal axis irradiation using helical tomotherapy, volume-modulated arc therapy and intensity-modulated radiotherapy for medulloblastoma. Transl Cancer Res 2019;8:191-202. [Crossref]
- Klonou A, Spiliotakopoulou D, Themistocleous MS, et al. Chromatin remodeling defects in pediatric brain tumors. Ann Transl Med 2018;6:248. [Crossref] [PubMed]
- Dunkel IJ, Gardner SL, Garvin JH Jr, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol 2010;12:297-303. [Crossref] [PubMed]
- Jiang C, Li X, Zhao H, et al. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer 2016;15:62. [Crossref] [PubMed]
- Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell 2007;129:1257-9. [Crossref] [PubMed]
- Deng J, Yang M, Jiang R, et al. Long Non-Coding RNA HOTAIR Regulates the Proliferation, Self-Renewal Capacity, Tumor Formation and Migration of the Cancer Stem-Like Cell (CSC) Subpopulation Enriched from Breast Cancer Cells. PLoS One 2017;12:e0170860. [Crossref] [PubMed]
- Taheri M, Habibi M, Noroozi R, et al. HOTAIR genetic variants are associated with prostate cancer and benign prostate hyperplasia in an Iranian population. Gene 2017;613:20-4. [Crossref] [PubMed]
- Hong Q, Li O, Zheng W, et al. LncRNA HOTAIR regulates HIF-1alpha/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis 2017;8:e2772. [Crossref] [PubMed]
- Wang W, He X, Zheng Z, et al. Serum HOTAIR as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Mol Cancer 2017;16:75. [Crossref] [PubMed]
- Chakravadhanula M, Ozols VV, Hampton CN, et al. Expression of the HOX genes and HOTAIR in atypical teratoid rhabdoid tumors and other pediatric brain tumors. Cancer Genet 2014;207:425-8. [Crossref] [PubMed]
- Fu WM, Lu YF, Hu BG, et al. Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget 2016;7:4712-23. [Crossref] [PubMed]
- Wang LP, Wang JP, Wang XP. HOTAIR contributes to the growth of liver cancer via targeting miR-217. Oncol Lett 2018;15:7963-72. [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Pepe F, Visone R, Veronese A. The Glucose-Regulated MiR-483-3p Influences Key Signaling Pathways in Cancer. Cancers (Basel) 2018;10:181. [Crossref] [PubMed]
- Ma J, Hong L, Xu G, et al. miR-483-3p plays an oncogenic role in esophageal squamous cell carcinoma by targeting tumor suppressor EI24. Cell Biol Int 2016;40:448-55. [Crossref] [PubMed]
- Huang X, Lyu J. Tumor suppressor function of miR-483-3p on breast cancer via targeting of the cyclin E1 gene. Exp Ther Med 2018;16:2615-20. [PubMed]
- Deng R, Yang D, Radke A, et al. The hypolipidemic agent guggulsterone regulates the expression of human bile salt export pump: dominance of transactivation over farsenoid X receptor-mediated antagonism. J Pharmacol Exp Ther 2007;320:1153-62. [Crossref] [PubMed]
- Kyrylkova K, Kyryachenko S, Leid M, et al. Detection of apoptosis by TUNEL assay. Methods Mol Biol 2012;887:41-7. [Crossref] [PubMed]
- Cai B, Song XQ, Cai JP, et al. HOTAIR: a cancer-related long non-coding RNA. Neoplasma 2014;61:379-91. [Crossref] [PubMed]
- Wang AH, Tan P, Zhuang Y, et al. Down-regulation of long non-coding RNA HOTAIR inhibits invasion and migration of oesophageal cancer cells via up-regulation of microRNA-204. J Cell Mol Med 2019;23:6595-610. [Crossref] [PubMed]
- Goodlad RA. Quantification of epithelial cell proliferation, cell dynamics, and cell kinetics in vivo. Wiley Interdiscip Rev Dev Biol 2017. [Crossref] [PubMed]
- Juríková M, Danihel L, Polak S, et al. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem 2016;118:544-52. [Crossref] [PubMed]
- Gu F, Li L, Yuan QF, et al. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis. Eur Rev Med Pharmacol Sci 2017;21:3504-9. [PubMed]
- Zheng H, Min J. Role of Long Noncoding RNA HOTAIR in the Growth and Apoptosis of Osteosarcoma Cell MG-63. Biomed Res Int 2016;2016:5757641. [Crossref] [PubMed]
- Yu Y, Lv F, Liang D, et al. HOTAIR may regulate proliferation, apoptosis, migration and invasion of MCF-7 cells through regulating the P53/Akt/JNK signaling pathway. Biomed Pharmacother 2017;90:555-61. [Crossref] [PubMed]
- Feng X, Huang S. Effect and mechanism of long noncoding RNAs HOTAIR on occurrence and development of gastric cancer. J Cell Biochem 2017. [Epub ahead of print]. [PubMed]
- Walther U, Emmrich K, Ramer R, et al. Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPARgamma-dependent pathway. Oncotarget 2016;7:10345-62. [Crossref] [PubMed]
- Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 2015;1219:1-9. [Crossref] [PubMed]
- Bleicken S, Hantusch A, Das KK, et al. Quantitative interactome of a membrane Bcl-2 network identifies a hierarchy of complexes for apoptosis regulation. Nat Commun 2017;8:73. [Crossref] [PubMed]
- Li X, Cheng T, He Y, et al. High glucose regulates ERp29 in hepatocellular carcinoma by LncRNA MEG3-miRNA 483-3p pathway. Life Sci 2019;232:116602. [Crossref] [PubMed]
- Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016;45:129-38. [Crossref] [PubMed]
- Cook Sangar ML, Genovesi LA, Nakamoto MW, et al. Inhibition of CDK4/6 by Palbociclib Significantly Extends Survival in Medulloblastoma Patient-Derived Xenograft Mouse Models. Clin Cancer Res 2017;23:5802-13. [Crossref] [PubMed]
- Bertero T, Gastaldi C, Bourget-Ponzio I, et al. CDC25A targeting by miR-483-3p decreases CCND-CDK4/6 assembly and contributes to cell cycle arrest. Cell Death Differ 2013;20:800-11. [Crossref] [PubMed]
- Bertero T, Bourget-Ponzio I, Puissant A, et al. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle 2013;12:2183-93. [Crossref] [PubMed]
- Zhou J, Wu Z, Wong G, et al. CDK4/6 or MAPK blockade enhances efficacy of EGFR inhibition in oesophageal squamous cell carcinoma. Nat Commun 2017;8:13897. [Crossref] [PubMed]
- Song X, Liu X, Wang H, et al. Combined CDK4/6 and Pan-mTOR Inhibition Is Synergistic Against Intrahepatic Cholangiocarcinoma. Clin Cancer Res 2019;25:403-13. [Crossref] [PubMed]
- Lee T, Kim K, Lee J, et al. Antitumor activity of sorafenib plus CDK4/6 inhibitor in pancreatic patient derived cell with KRAS mutation. J Cancer 2018;9:3394-9. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


