
Aging aggravated liver ischemia and reperfusion injury by promoting hepatocyte necroptosis in an endoplasmic reticulum stress-dependent manner
Introduction
IR injury is a fundamental obstacle for the recovery of patients who undergo liver resection and transplantation surgery. The number of elderly patients that receive liver surgeries is increasing as the population ages. Organ shortage represents an important social and healthcare issue, and organ donations after cardiac death (DCD) from elderly individuals have been considered a vital means to fill the pool of liver grafts. Thus, it is important to study how to alleviate aged liver IR injury and to optimize the use of aged liver grafts.
Necroptosis, a type of programmed cell death, is characterized by the disruption of the plasma membrane, lysis of a swollen cell and release of damage-associated molecular patterns (DAMPs). Necroptosis is widely involved in age-related diseases (1,2). The increased expressions of phosphorylated mixed lineage kinase domain like pseudokinase (P-MLKL) and MLKL, and elevated inflammatory cytokines were found in the epididymal white adipose tissue of aged mice even under resting conditions (3,4), which indicated that aged mice tended to initiate necroptosis and inflammation.
Necroptosis activation has been found in many models of diseases, including IR. Several studies have demonstrated that anti-necroptosis treatment protected against IR injury in the kidney (5), cerebrum (6) and retina (7), but whether anti-necroptosis treatment could protect the liver from IR injury remains controversial. Necroptosis inhibition by suppressing receptor-interacting protein kinase 1 (RIP1) had few protective effects, Saeed et al. considered that necroptosis activation was dispensable for liver IR injury (8). Another study showed that RIP3 depletion protected the liver from IR injury at the late phase (24 hours after perfusion) and that MLKL knockout (KO) protected young mice from liver IR at both the early (6 hours after reperfusion) and late phases (9). Our previous study showed that RIP1 inhibition reduced prolonged liver IR-induced Kupffer cell necrotic depletion and thus alleviated liver injury (10). In aged mice, necroptosis inhibition significantly relieved heart IR injury, indicating the key role of necroptosis in promoting aging-induced intolerance to IR injury (11). However, limited data about the role of necroptosis in aged mice post liver IR are available.
The endoplasmic reticulum (ER) plays crucial roles in cellular function and homeostasis by facilitating protein folding. During the liver IR process, the function of protein folding is disturbed, resulting in quantities of misfolded proteins accumulated in the ER, which is known as ER stress. Previously, it was found that ER stress inhibition by 4-PBA significantly alleviated liver IR injury in young mice (12). Upregulated ER stress was revealed in aged mammals. It was reported that excessive oxidative stress increased ER stress in aged mice, leading to exacerbated tunicamycin-induced acute kidney injury (13). Kim et al. found age-associated dyslipidemia and hyperglycemia upregulated ER stress, which promoted liver lipogenesis and steatosis in aged rats (14). Increased ER stress indicated by higher levels of glucose-regulated protein 78 (GRP78), cleaved-activating transcription factor 6 (ATF6) and x-box binding protein1 were observed in the aged adipose stromal cells, which further contributed to adipose tissue inflammation in aged mice (15). However, little is known about ER stress in aged mice post liver IR.
Interestingly, the role of ER stress in regulating necroptosis was reported recently. In lipopolysaccharide (LPS) or H2O2-treated cardiomyocytes, upregulated RIPK3 contributed to calcium overload, xanthine oxidase and reactive oxygen species (ROS) production and mitochondrial permeability transition pore opening, which led to increased cardiomyocyte necroptosis (16). However, the effects of ER stress on necroptosis post liver IR remain unclear.
In this study, we investigated whether and how necroptosis affected hepatic IR injury in aged mice. We demonstrated that IR triggered excessive ER stress in aged livers, leading to hepatocyte necroptosis and eventually aggravating liver injury.
Methods
Mice
Male C57BL/6 mice aged 8 weeks (young mice) and 100 weeks (aged mice) were purchased from GemPharmatech Co., Ltd. (Nanjing, China). The weight (± SEM) of the young group was 26.7±0.22 grams, while that of the aged mice was 29.2±0.33 grams. The mice were maintained under specific pathogen-free conditions with free access to water and standard chow with supplements. The mice received humane care in compliance with a protocol (protocol number NMU08-092) approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. All animal work abided by the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Model of warm liver IR
A model of segmental hepatic warm IR was used following the protocol described previously (17). In brief, after the mice were successfully anesthetized with inhaled isoflurane (1.5%), an atraumatic clip was used to block the arterial and portal venous blood supply to the hepatic cephalad lobes. The clip was removed to initiate liver reperfusion after 90 minutes of segmental hepatic ischemia. The mice were sacrificed 6 hours after reperfusion. Sham controls underwent the same procedure but without vascular occlusion. Nec-1 (1 mg/kg; Enzo Life Science, New York City, USA), 4-PBA (100 mg/kg; Sigma, St. Luis, USA), or PBS (vehicle control) was administered intraperitoneally 3 hours before the onset of liver ischemia.
Serum biochemical measurements and liver histopathology
Serum alanine aminotransferase (ALT) and aspartate transaminase (AST) were measured with an AU680 clinical chemistry analyzer (Beckman Coulter, Atlanta, USA). Liver specimens were fixed in 4% paraformaldehyde, embedded in paraffin and stained with hematoxylin and eosin (H&E). The severity of liver IR injury was quantified using the Suzuki score. Tissues without necrosis or congestion/centrilobular ballooning were given a score of 0, while tissues presenting with severe congestion and/or >60% lobular necrosis were given a score of 4.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA (2 µg) was reverse transcribed into cDNA using an RR047A PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Japan). qRT-PCR was performed with a StepOnePlus Real-Time PCR system (Thermo Fisher Scientific, Waltham, USA) in a final reaction volume of 20 µL, containing 1× TB Green Premix (TaKaRa, Japan), complementary DNA, and each primer at 0.125 µM. The amplification conditions were as follows: 50 °C for 2 minutes; 95 °C for 10 minutes; and 40 cycles of 95 °C for 15 s and 60 °C for 1 minutes. The following primers were used: ATF4 forward (Fwd): 5'-ATGGCGCTCTTCACGAAATC-3', reverse (Rev): 5'-ACTGGTCGAAGGGGTCATCAA-3'; ATF6 Fwd: 5'-GGACGAGGTGGTGTCAGAG-3', Rev: 5'-GACAGCTCTTCGCTTTGGAC-3'; GRP78 Fwd: 5'-CCTCTCTGGTGATCAGGATA-3', Rev: 5'-CGTGGAGAAGATCTGAGACT-3'; GAPDH Fwd: 5'-CATGTTCCAGTATGACTCCACTC-3', Rev: 5'-GGCCTCACCCCATTTGATGT-3'.
Western blotting
Liver tissue proteins were extracted with ice-cold lysis buffer. Proteins (30 µg) were subjected to 10% SDS-PAGE electrophoresis and transferred to PVDF nitrocellulose membranes. Antibodies against RIP1, P-RIP3 Ser232, P-MLKL Ser345, ATF6 p50, ATF4, GRP78 and β-actin (Cell Signaling Technology, Danvers, USA) were used for Western blot analysis. Densitometry to determine changes in protein expression was measured using ImageJ software.
Statistical analysis
Quantitative values were presented as the mean ± SEM. Multiple group comparisons were performed using one-way analysis of variance ANOVA followed by Bonferroni's post hoc test. All analyses were performed using GraphPad 8.0. P values <0.05 (two-tailed) were considered statistically significant.
Results
Aged mice presented with aggravated liver injury post hepatic IR
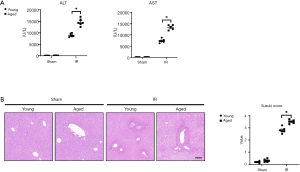
Young and aged mice were subjected to partial warm ischemia of the liver followed by 6 hours of reperfusion. The extent of liver injury and inflammation was compared between the groups. Serum AST and ALT levels were higher in aged mice after hepatic IR than in young mice (Figure 1A, IR: aged vs. young), and aged mice showed worsened damage to histological liver architecture as shown by H&E staining and higher Suzuki scores (Figure 1B, IR: aged vs. young). We found that aged mice presented with aggravated liver injury after IR.
Aging aggravated liver IR injury by enhancing hepatocyte necroptosis
Whether necroptosis is involved in liver IR injury remains controversial. Several studies considered that necroptosis was dispensable for liver IR injury (8,18). Other researchers hold the opposite view, they found that necroptosis inhibition could relieve liver IR injury (9). Our previous study showed that RIP1 inhibition reduced prolonged liver IR-induced Kupffer cell necrotic depletion and alleviated liver damage (10). Here, we evaluated necroptosis activation in the aged liver post-IR. We found slightly increased expressions of RIP1 and P-RIP3 in the young mice post-IR (Figure 2A, Sham young vs. IR young) but significantly enhanced expression of necroptosis-related proteins in aged livers (Figure 2A, IR: aged vs. young).
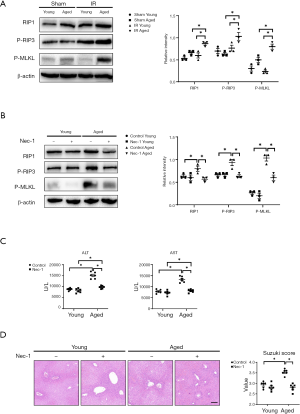
To further study the role of necroptosis in liver IR injury, Nec-1, a specific RIP1 inhibitor, was used 3 hours before liver partial warm IR. Nec-1 pretreatment efficiently suppressed IR-induced necroptosis in aged mice (Figure 2B, Nec-1 aged vs. control aged), Nec-1 pretreatment protected aged livers from IR injury as evidenced by decreased serum AST and ALT (Figure 2C, Nec-1 aged vs. control aged), hepatic injury mitigation and decreased Suzuki score (Figure 2D, Nec-1 aged vs. control aged). However, Nec-1 pretreatment had no protective effects on young mice (Figure 2B,C,D, Nec-1 young vs. control young). In brief, Nec-1 pretreatment alleviated liver IR injury in aged mice, but not in young mice, by suppressing hepatocyte necroptosis.
Overactivation of ER stress in aged mice post hepatic IR
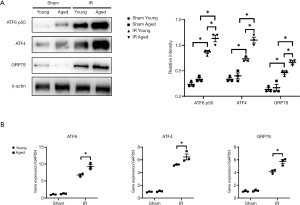
ER stress is implicated in the pathogenesis of liver IR injury in young mice (10), and our study analyzed ER stress activation in aged mice post liver IR. Western blot analysis demonstrated elevated expressions of ATF6 p50, ATF4 and GRP78 in both young and aged mice after IR (Figure 3A, IR young vs. sham young; IR aged vs. IR aged) and enhanced ER stress in aged mice (Figure 3B, IR: aged vs. young). The same results were observed at the transcriptional level (Figure 3B, IR young vs. sham young; sham aged vs. IR aged; IR: aged vs. young). Thus, we found that increased ER stress was induced by liver IR in aged mice.
4-PBA pretreatment attenuated hepatocyte necroptosis in aged mice by suppressing ER stress
Our previous study demonstrated that ER stress inhibition by 4-PBA effectively alleviated liver IR injury in young mice (12). In this study, we tested whether 4-PBA pretreatment had protective effects on aged mice. Mice were intraperitoneally injected with 4-PBA 3 hours before liver partial warm IR. 4-PBA administration efficiently suppressed IR-induced ER stress and alleviated liver injury in both young and aged mice (Figure 4A,B,C, 4-PBA young vs. control young; 4-PBA aged vs. control aged). Notably, the protective effects of 4-PBA pretreatment were more significant in aged mice, leading to comparable liver IR injury in young and aged mice after IR (Figure 4B,C, 4-PBA young vs. 4-PBA aged).
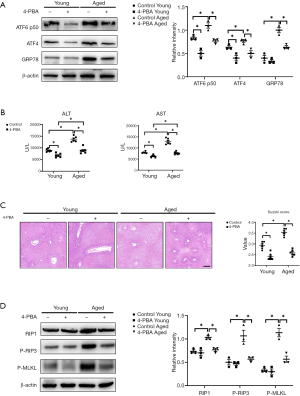
In addition, ER stress blockade by 4-PBA further suppressed hepatocyte necroptosis in aged mice, as evidenced by decreased expression of RIP1, P-RIP3, and P-MLKL, but not in young mice (Figure 4D, 4-PBA aged vs. control aged). However, 4-PBA pretreatment had feeble a weak influence on necroptosis-related protein expressions in young mice, which indicated that 4-PBA administration protected against liver IR in young mice independent of necroptosis signaling (Figure 4D, 4-PBA young vs. control young). Taken together, these results suggest that 4-PBA pretreatment attenuated hepatocyte necroptosis and liver IR injury in aged mice by suppressing ER stress.
Discussion
In our study, we demonstrated for the first time that aging aggravated liver IR injury by promoting hepatocyte necroptosis in an ER stress-dependent manner. Necroptosis inhibition by Nec-1 mitigated hepatocyte necroptosis and liver IR injury in the aged group but not in the young group. ER stress inhibition by 4-PBA alleviated hepatocellular necroptosis and protected aged mice from liver IR injury in aged mice.
Necroptosis is one kind of programmed death characterized by the disruption of the plasma membrane, lysis of the swollen cell and the release of DAMPs. Severe necroptosis results in a large amount of cell death and excessive inflammation activation, and thus ultimately aggravates damage to the body. Generally, necroptosis is controlled by RIP1, RIP3 and MLKL. Several studies demonstrated that anti-necroptosis treatment has protective effects on IR injury of the kidney (5), cerebrum (6) and retina (7). Whether necroptosis inhibition could alleviate liver IR injury remains controversial. Rosentreter et al. found that IR-induced caspase activation led to the cleavage of RIP1; thus, RIP1-mediated necroptosis was not present in IR-stressed livers (18). Saeed et al. showed no changes in RIP1 and RIP3 expression post liver IR compared to the sham group in young mice, and RIP1 inhibition by Nec-1, an efficient RIP1 inhibitor, did not protect against liver IR injury in young mice (8). Our previous study showed that RIP1 inhibition reduced prolonged liver IR-induced Kupffer cell necrotic depletion and thus alleviated inflammation activation and liver damage (10). It was showed that necroptosis inhibition by Nec-1 decreased extracellular signal-regulated kinase activation and attenuated liver IR injury (10). Another study demonstrated that RIP3 KO mitigated IR injury in young mouse livers at the late phase (24 hours post reperfusion), and MLKL KO protected young mice from liver IR at 6 and 24 hours post liver IR (9). Additionally, few studies have evaluated the role of necroptosis in aged mice post liver IR.
Necroptosis is widely implicated in age-related diseases, such as Alzheimer’s disease (19), amyotrophic lateral sclerosis (20), age-related macular degeneration (21), atherosclerosis (22), and cancer (1). Previous studies found that aged mice presented with aggravated intrahepatic inflammation and injury post liver IR (23), but the mechanism was not well understood. Age-related anatomical alterations and functional declines in the liver made aged liver more vulnerable in response to IR stress. Aging-related deteriorations in the immune system disrupted the balance between pro- and anti-inflammatory responses, leading to a basal chronic, low-grade proinflammatory state, which is referred to as “inflammaging” (24,25). Increased expression of P-MLKL and MLKL, and elevated inflammatory cytokines were found in the epididymal white adipose tissue of aged mice even under resting states (3,4), indicating the tendency of necroptosis and inflammation initiation in aged mice. Recently, Li et al. reported that metformin administration protected aged mice from heart IR injury by suppressing necroptosis (11), indicating that necroptosis was crucially involved in aging-induced intolerance to IR injury. Here, our study demonstrated that Nec-1 reduced hepatocellular necroptosis and protected against liver IR injury in aged mice.
Necroptosis is also involved in other models of liver injury. It was found that RIP or RIP3 knockdown by specific siRNA significantly relieved Con A-induced hepatitis by mitigating necroptosis and PARP-1-dependent intracellular ATP depletion (11). In an APAP-induced acute liver injury model, Deutsch et al. found that RIP3 KO only had a limited protective effect with no survival advantages compared to WT, but RIP1 knockdown by Nec-1 significantly diminished hepatocyte injury (26). In contrast, RIP1 inhibition by Nec-1 showed no protective effects on alcoholic-induced chronic liver injury, but RIP3 KO effectively ameliorated liver steatosis and hepatocyte damage independent of RIP1 kinase activity. These data indicated that RIP1 or RIP3 inhibition could have different effects on tissue damage.
The ER is an important organelle in maintaining cellular function and homeostasis. During the IR process, quantities of misfolded proteins accumulated in the ER due to hypoxia and oxidative stress, as well as ion imbalance. Protein misfolding disorders are associated with many age-related diseases, such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease (2). Our previous studies found that ER stress inhibition by 4-PBA significantly alleviated liver IR injury in young mice (12). Upregulated ER stress was revealed in aged mammals. Liu et al. found that excessive oxidative stress increased ER stress in aged mice, leading to exacerbated tunicamycin-induced acute kidney injury (13). It was reported that age-associated dyslipidemia and hyperglycemia upregulated ER stress, which promoted liver lipogenesis and steatosis in aged rats (14). Increased ER stress indicated by higher levels of GRP78, cleaved ATF6 and x-box binding protein1 were observed in the aged adipose stromal cells, which further contributed to adipose tissue inflammation in aged mice (15). Targeting ER stress would be a potential therapy, however, there are few reports about ER inhibition in regulating aged liver IR injury.
Recently, accumulated studies described the interaction between ER stress and necroptosis. GRP78 and P-MLKL were found to be coexpressed in human microglia post spinal cord injury. Furthermore, ER stress inhibition by 4-PBA efficiently mitigated oxygen-glucose deprivation induced microglia necroptosis (27). In L929 cells, brefeldin-induced ER stress was reported to activate necroptosis signaling, dependent on tumor necrosis factor receptor 1 (TNFR1) but independent of the participation of ligands (28). These results indicated that ER stress activation contributed to necroptosis. On the other hand, Ma et al. reported that necroptosis of human lung cancer cells promoted ROS-induced ER stress through CHOP activation and nuclear factor-kappa B (NF-kB) inhibition (29). In H2O2-treated cardiomyocytes, Zhu et al. found that remarkably upregulated RIP3 evoked ER stress activation, which further induced cardiomyocyte necroptosis in a calcium overload/XO/ROS/mPTP-opening manner (16). In our previous study, we found that autophagy played a critical role in mediating ER stress-induced hepatocellular injury/death (12). Here, we found upregulated hepatocellular ER stress and necroptosis post liver IR in aged mice, and 4-PBA treatment alleviated IR-induced necroptosis. However, the precise mechanism of ER stress in regulating hepatocellular autophagy and necroptosis remains to be further studied.
In summary, aging exacerbated ER stress in IR-stressed hepatocytes, which led to aggravated necroptosis and liver injury. Our study demonstrated a novel mechanism of ER stress in the regulation of necroptosis in aged livers in response to IR, which would be a potential therapeutic target to reduce liver IR injury in elderly patients.
Acknowledgments
Funding: This work was supported by grants from National Nature Science Foundation of China (81530048, 81870448, 31930020, 81600450, 81901628), the National Science Foundation of Jiangsu Province (BK20191490), CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-035), Six Talent Peaks Project in Jiangsu Province (No. 2018-WSN-011), Jiangsu Science and Technology Association Young Science and Technology Talents Lifting Project (No. DG000D4007) and A Project Funded by the PAPD.
Footnote
Data Sharing Statement: Available http://dx.doi.org/10.21037/atm-20-2822
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2822). XW serves as an unpaid editorial board member of Annals of Translational Medicine from Aug 2019 to Jul 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The mice received humane care in compliance with a protocol (protocol number NMU08-092) approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. All animal work abided by the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gong Y, Fan Z, Luo G, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer 2019;18:100. [Crossref] [PubMed]
- Martínez G, Duran-Aniotz C, Cabral-Miranda F, et al. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 2017;16:615-23. [Crossref] [PubMed]
- Deepa SS, Unnikrishnan A, Matyi S, et al. Necroptosis increases with age and is reduced by dietary restriction. Aging Cell 2018;17:e12770. [Crossref] [PubMed]
- Royce GH, Brown-Borg HM, Deepa SS. The potential role of necroptosis in inflammaging and aging. Geroscience 2019;41:795-811. [Crossref] [PubMed]
- Linkermann A, Brasen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2013;110:12024-9. [Crossref] [PubMed]
- Nikseresht S, Khodagholi F, Ahmadiani A. Protective effects of ex-527 on cerebral ischemia-reperfusion injury through necroptosis signaling pathway attenuation. J Cell Physiol 2019;234:1816-26. [Crossref] [PubMed]
- Rosenbaum DM, Degterev A, David J, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res 2010;88:1569-76. [PubMed]
- Saeed WK, Jun DW, Jang K, et al. Does necroptosis have a crucial role in hepatic ischemia-reperfusion injury? PLoS One 2017;12:e0184752. [Crossref] [PubMed]
- Ni HM, Chao X, Kaseff J, et al. Receptor-Interacting Serine/Threonine-Protein Kinase 3 (RIPK3)-Mixed Lineage Kinase Domain-Like Protein (MLKL)-Mediated Necroptosis Contributes to Ischemia-Reperfusion Injury of Steatotic Livers. Am J Pathol 2019;189:1363-74. [Crossref] [PubMed]
- Yue S, Zhou H, Wang X, et al. Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J Immunol 2017;198:3588-95. [Crossref] [PubMed]
- Li C, Mu N, Gu C, et al. Metformin mediates cardioprotection against aging-induced ischemic necroptosis. Aging Cell 2020;19:e13096. [Crossref] [PubMed]
- Zhou H, Zhu J, Yue S, et al. The Dichotomy of Endoplasmic Reticulum Stress Response in Liver Ischemia-Reperfusion Injury. Transplantation 2016;100:365-72. [Crossref] [PubMed]
- Liu X, Zhang R, Huang L, et al. Excessive Oxidative Stress Contributes to Increased Acute ER Stress Kidney Injury in Aged Mice. Oxid Med Cell Longev 2019;2019:2746521. [PubMed]
- Kim DH, Ha S, Choi YJ, et al. Altered FoxO1 and PPARgamma interaction in age-related ER stress-induced hepatic steatosis. Aging (Albany NY) 2019;11:4125-44. [Crossref] [PubMed]
- Ghosh AK, Garg SK, Mau T, et al. Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci 2015;70:1320-9. [Crossref] [PubMed]
- Zhu P, Hu S, Jin Q, et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: A mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 2018;16:157-68. [Crossref] [PubMed]
- Zhou H, Wang H, Ni M, et al. Glycogen synthase kinase 3beta promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J Hepatol 2018;69:99-109. [Crossref] [PubMed]
- Rosentreter D, Funken D, Reifart J, et al. RIP1-Dependent Programmed Necrosis is Negatively Regulated by Caspases During Hepatic Ischemia-Reperfusion. Shock 2015;44:72-6. [Crossref] [PubMed]
- Ofengeim D, Mazzitelli S, Ito Y, et al. RIPK1 mediates a disease-associated microglial response in Alzheimer's disease. Proc Natl Acad Sci U S A 2017;114:E8788-97. [Crossref] [PubMed]
- Ito Y, Ofengeim D, Najafov A, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016;353:603-8. [Crossref] [PubMed]
- Murakami Y, Matsumoto H, Roh M, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ 2014;21:270-7. [Crossref] [PubMed]
- Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci U S A 2015;112:11007-12. [Crossref] [PubMed]
- Jiang T, Zhan F, Rao Z, et al. Combined ischemic and rapamycin preconditioning alleviated liver ischemia and reperfusion injury by restoring autophagy in aged mice. Int Immunopharmacol 2019;74:105711. [Crossref] [PubMed]
- Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood 2005;105:2294-9. [Crossref] [PubMed]
- Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging Dis 2011;2:346-60. [PubMed]
- Deutsch M, Graffeo CS, Rokosh R, et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis 2015;6:e1759. [Crossref] [PubMed]
- Fan H, Tang HB, Kang J, et al. Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience 2015;311:362-73. [Crossref] [PubMed]
- Saveljeva S, Mc Laughlin SL, Vandenabeele P, et al. Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis 2015;6:e1587. [Crossref] [PubMed]
- Ma YM, Peng YM, Zhu QH, et al. Novel CHOP activator LGH00168 induces necroptosis in A549 human lung cancer cells via ROS-mediated ER stress and NF-kappaB inhibition. Acta Pharmacol Sin 2016;37:1381-90. [Crossref] [PubMed]


