A single-center 14-year follow-up study of the BalMedic® bovine pericardial bioprosthetic valve
Introduction
With the advancement of valve leaflets processing technology and valve structure optimization, the durability and effectiveness of bioprosthetic valves have been greatly improved. Bioprosthetic valves have become the preferred choice for valve replacement in developed countries in Europe and North America. Many clinical follow-up studies have demonstrated excellent clinical effectiveness of bioprosthetic valves (1,2). The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines and the 2015 European Society of Cardiology (ESC) Guidelines have expanded the clinical populations indicated for bioprosthetic valve replacement and, for the first time, lowered to the age group of 50 (3). With the aging of society, advanced age, coronary heart disease, and myocardial infarction are becoming common causes of valvular disease in China. Moreover, the prevalence of rheumatic heart disease caused by hemolytic streptococcus is high in rural and suburban areas in China, and patients with rheumatic heart disease who have progressed to moderate to severe valvular stenosis and regurgitation also require valve replacement (4).
Patients with rheumatic heart disease are relatively young to those with degenerative disease. Due to concerns about the durability of bioprosthetic valves, mechanical valves are used in most cases of valve replacement in this age group, which leads to the low usage rate of bioprosthetic valves in China in the past and the lack of large-scale, long-term follow-up data on postoperative outcomes of bioprosthetic valves. In recent years, with the increasing use of imported bioprosthetic valves, some surgical centers have started to focus on long-term follow-up in Chinese patients after bioprosthetic valve replacement and have published follow-up data on some foreign products, including survival and postoperative complications (5). Beijing Balance Medical Tech Co., Ltd. is the first manufacturer of bovine pericardial bioprosthetic valves in China, and their products incorporate unique, patented anticalcification technology. Bovine pericardial materials treated with this technology have been widely accepted and used for a long time in patients with congenital heart disease and have demonstrated the effectiveness of the anticalcification technology. Since BalMedic bovine pericardial bioprosthetic valves were approved by the China Food and Drug Administration (CFDA) in 2003, more than 10,000 valves have been implanted at over 400 hospitals (with documentation) in China. The Department of Cardiac Surgery of The First People’s Hospital of Yulin is one of the first facilities to use BalMedic valves.
In this study, we analyzed the follow-up data of patients undergoing BalMedic bovine pericardial bioprosthetic valve replacement at our hospital over a 9-year span. According to surgical records, a bioprosthetic valve(s) was used in 59% of valve replacements during this period. To date, this is the largest single-center follow-up study and one of the largest long-term (10 years or more) follow-up studies on BalMedic bovine pericardial bioprosthetic valves in China. The results provide important evidence for clinical evaluation of the durability of BalMedic bioprosthetic valves.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3790).
Methods
This study was reviewed and approved by the Ethics Committee of The First People’s Hospital of Yulin. The tissue valves implanted are all products obtained premarket approval from CFDA (China Food and Drug Administration).
We searched hospital management systems and medical records and collected clinical data of 299 patients undergoing BalMedic bovine pericardial bioprosthetic valve replacement [336 valves, including double valve replacement (DVR)] at our hospital between 2005 and 2014. That is all patients who underwent BalMedic valve production replacement in the meantime. During the follow-up period, we contacted the patients or their family members to obtain information on survival and the second surgery. Surviving patients and at least one BalMedic valve after the second surgery underwent electrocardiogram (ECG) and echocardiography. Most patients made an appointment on the phone to return to our hospital for follow-up visits, and some patients followed up at local hospitals with hospital reports. For patients unable to return to a hospital for follow-up visits due to age, economic condition, limited mobility, or unwillingness, the study staff visited these patients at home to perform ECG and echocardiography. Five patients were lost to follow-up due to loss of contact, while 98.28% of the patients (285/290) completed the follow-up. The mean age was 53.50±10.0 years, and the mean follow-up time was 7.7±2.5 years (5 to 14). Subgroup analyses were performed per surgical approach, including aortic valve replacement (AVR), mitral valve replacement (MVR), and double valve replacement(DVR); or age, including <50, ≥50 and <60, ≥60 and <70, and ≥70 years.
Study endpoints
The primary endpoints were death and reoperation. The secondary endpoint was structural valve damage deterioration (SVD). SVD in this study was defined as valve open/closure dysfunction due to valve calcification, tearing, or degenerative changes and was evaluated with echocardiography during the follow-up period. At our hospital, SVD was diagnosed as any valve dysfunction or failure for reasons other than infection and thrombosis. Due to limitations in the original records, we did not perform detailed analysis of other valve-related complications, such as hemorrhage, embolism, infective endocarditis, and nonstructural valve dysfunction (such as paravalvular leakage).
Statistical analysis
SAS v9.4 was used for statistical analysis. Continuous variables are expressed as case number, mean, standard deviation (SD), minimum, and maximum. Categorical variables are expressed as frequency and percentage. Survival data are expressed as median, and the Kaplan-Meier method was used to estimate survival rates. The log-rank test was performed to compare survival curves. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
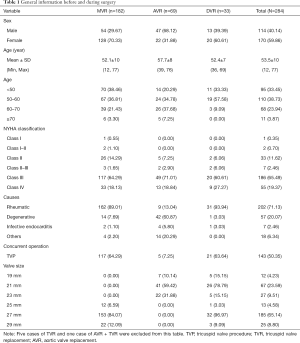
A total of 299 patients underwent BalMedic bovine pericardial bioprosthetic valve replacement at our hospital between 2005 and 2014. Among them, 290 patients were discharged, and their data were included in this study. Five patients underwent tricuspid valve replacement (TVR), and one underwent AVR combined with TVR. These patients were not assigned to any study group due to low case numbers. Finally, 284 patients were divided into three groups, and Table 1 shows their general information before and during surgery. 69(24.30%) patients underwent AVR, 182(64.08%) underwent MVR, and 33 (11.62%) underwent DVR. The patients were relatively young (53.50±10), 71.13% of them had rheumatic heart disease, and 20.07% had degenerative valve disease. Before surgery, 84.86% of the patients belonged to New York Heart Association (NYHA) functional class III and above. Concurrent operation was mainly the tricuspid valve procedure (TVP; n=143). A total of 317 valves were implanted in these 284 patients, including 215 (67.8%) mitral valves and 102 (32.2%) aortic valves. The main size of mitral valve used was 27 mm (84%), and the main sizes of aortic valve used were 21 mm (59%) and 23 mm (32%).
Full table
Postoperative mortality
Nine patients died in the hospital within 30 days after surgery (perioperative period). Thus, the early mortality rate was 3% (9/299), including five patients in the MVR group, one patient in the AVR group, and three patients in the DVR group. A total of 290 patients survived to discharge. Five patients were lost to follow-up and were excluded from study. Six patients declined follow-up visits; given their known survival status, they were included in survival analysis and were counted in statistics of the second surgery but were excluded from SVD statics and analysis. During the follow-up period, 68 patients (23.4%, 68/290) died, including 48 patients (26.4%; 48/182) in the MVR group, 13 (18.8%; 13/69) in the AVR group, and 5 (15.2%; 5/33) in the DVR group, the rest 2 cases are TVR involved. Twenty-two deaths were cardiac (details unknown), 25 were non-cardiac, and 21 were of unknown cause (not recorded).
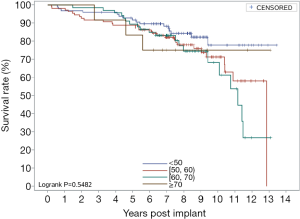
Figure 1 shows overall survival curves, and the 5- and 10-year survival rates were 89.95% and 72.53%, respectively. Figure 2 shows the survival of the AVR group, the MVR group, and the DVR group. Their respective 5-year survival rates were 93.98%, 88.40%, and 90.00%, and the 10-year survival rates were 80.64%, 67.21%, and 82.90%. Figure 3 shows the survival of different age groups (<50, 50–60, 60–70, ≥70). Their respective 5-year overall survival rates were 91.7%, 88.92%, 89.55%, and 83.33%, and the 10-year overall survival rates were 77.86%, 71.34%, 68.21%, and 75%. Subgroup analyses by surgical approach or age showed no significant intergroup differences in overall survival.

Second surgery
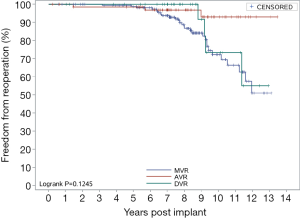
Figure 4 shows the overall reoperation-free rates. The 5- and 10-year reoperation-free rates were 98.86% and 77.47%, respectively. Figure 5 shows the reoperation-free rates in the AVR group, the MVR group, and the DVR group. AVR demonstrated sustained results, with 5- and 10-year reoperation-free rates of 98.51% and 92.94%, respectively. MVR and DVR demonstrated similar results. For MVR, the 5- and 10-year reoperation-free rates were 98.78% and 72.26%, respectively; for DVR, the rates were 100% and 73.33%. Figure 6 shows the reoperation-free rates in different age groups. The patients in this study were relatively young, and most underwent MVR. As a result, the 5- and 10-year reoperation-free rates were significantly lower in patients aged below 60 than in patients aged 60 or above. There were no differences between the three surgery groups in reoperation-free rate, but there were significant differences between the age groups.
SVD
SVD has been used as an important criterion for second surgery, but it has not yet been clearly defined. As a result, some patients with SVD may not need second surgery. SVD reflects the overall impact of postoperative events that may cause valve failure. In this study, the diagnostic criteria for SVD of the aortic valve were as follows: moderate to severe regurgitation, mean transvalvular pressure >40 mm Hg, or peak flow >3.8 m/s. The diagnostic criteria for SVD of the mitral valve were as follows: moderate to severe regurgitation, mean transvalvular pressure >8 mmHg or maximum transvalvular pressure >25 mmHg, or peak flow >2.5 m/s. These criteria were in line with those used in most studies.
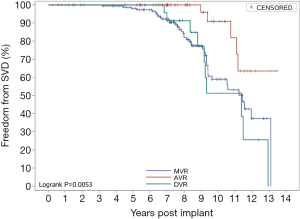
Figure 7 shows the overall SVD-free rates. The implanted valves were generally stable during the first 5 years after surgery (5-year SVD-free rate: 98.8%), but the 10-year SVD-free rate was 65.76%. Figure 8 shows the SVD-free rate in the AVR group, the MVR group, and the DVR group respectively. In the AVR group, the 10-year SVD-free rate was 90.95%, which was significantly higher than that in the MVR group (58.90%) and the DVR group (53.83%). The 10-year SVD-free rate was similar between the MVR group and the DVR group, because all SVD events in the DVR group occurred in the mitral valve, not the aortic valve. Figure 9 shows the SVD-free rates in different age groups. Like the reoperation-free rates, in patients aged below 70, the SVD-free rate was lower in younger patients than that in older patients, and the trend was even observed in 5-year data. Whereas the 5-year SVD-free rate was 100% in the patients in other age groups, it was 94.97% in MVR patients aged below 50 and further fell to 42.14% by year 10 after valve replacement. In other words, more than half of younger MVR patients had varying degrees of SVD by year 10 after valve replacement. Similar trends were observed in the AVR group and the DVR group, showing significantly higher incidences of SVD in patients aged below 50 by year 10 after valve replacement.

Discussion
Anticoagulation-related complications affect the durability of mechanical valves (6), while the treatments of valve leaflets with animal-derived materials play a key role in the lifespan of bioprosthetic valves. Each manufacturer has extended the life of bioprosthetic valves with its own biochemical treatment technology (7). However, postimplant bioprosthetic valve failure, especially process-related calcification, is always a vexing issue. Since the glutaraldehyde process for bovine pericardial materials was introduced in the 1970s, it has largely improved the strength of valve leaflets by more collagen cross-linking. On the other hand, it also increases the risk of calcification because excessive free negative radical groups are exposed (8).
BalMedic bovine pericardial valves (Beijing Balance Medical Tech Co., Ltd.) are manufactured with a novel and innovative process that introduces a coordination compound with a free positive group for the cross-link task. It will strengthen the leaflet material and lower down the risk of calcification simultaneously. The effect has been clinically proved in different surgical patches, as well as congenital heart disease implantation where needs the highest requirement for anti-calcification (9,10).
BalMedic bovine pericardial valve obtained CFDA marketing approval in 2003. Since then, it has been implanted in more than 10,000 patients. This study is the largest long-term retrospective follow-up clinical data of the production since market authorization. Preoperative data showed that rheumatic heart disease was the leading cause of valvular disease (>70%), more than 70% of the patients underwent MVR or DVR. This differs greatly from the cases in the Europe and North America, where most patients undergo AVR due to degenerative valve disease. Because patients with rheumatic heart disease are relatively young, the main feature of the group is younger patients underwent MVR. Moreover, 84.86% of the patients were classified as NYHA class III or above, and 19.37% were diagnosed as NYHA class IV before valve replacement, suggesting a worse preoperative cardiac condition in Chinese patients than in European and American patients (11-13).
Even considering the level of healthcare service, the severity of patient condition at first visit, patient awareness of self-care, and economic conditions in China, survival analysis showed that the 10-year survival rate is satisfied compared to its foreign counterparts on average, especial in AVR and DVR (12-15). The more comparable result is from Chinese local patients replaced with Perimount bovine pericardial bioprosthetic valves (14). In this study, the 5-year overall survival rate of AVR/MVR/DVR were 81.58%, 86.46% and 74.42% respectively. Ten-year data were 66.19%, 64.39%, and 55.85%. Except MVR results are about the same, AVR/DVR results of present study are all 10 percent higher at least, which indicates obvious advantages.
The reoperation rate in this study was significantly higher in young patients (below 50) than in older patients (50 or above). Subgroup analyses by surgical approach (AVR, MVR, DVR) and age demonstrated the same trend. Until recently, the guidelines advised against the use of bioprosthetic valves in patients younger than 50. As a result, follow-up data in young patients undergoing bioprosthetic valve replacement are lacking.
Each center has its own diagnostic criteria, which leads to variation in SVD diagnosis (16). Further progression of SVD could lead to second valve replacement or even death. This study showed that no SVD happened within 5 years after valve replacement. While for 10 years results, nearly two-thirds of patients aged below 50 developed SVD, which were significantly more than those in patients aged 50 or above. Subgroup analysis per surgical approach showed similar trends in reoperation rate between patients aged below 50 and patients aged 50 or above. These data indicate that SVD and reoperation rates are highly correlated with age.
In this study, the follow-up lasted up to 14 years (mean: 7.7±2.5), making it an intermediate- to long-term follow-up. Several large long-term follow-up studies abroad have analyzed the patients undergoing bioprosthetic valve replacement for 20 to 25 years (11,17,18). For older patients or patients with overall poor health, natural death or death due to other causes could affect the number of surviving patients during the follow-up period (19). Therefore, for longer-term follow-up studies, it is more meaningful to evaluate the life of bioprosthetic valves by distinguishing physical illnesses or deaths due to different causes. In this follow-up study, the mean age was 53.5. If the follow-up period were extended to 20 years, the mean age would reach the mean life expectancy in China, which means that natural mortality could have an impact on patient survival, resulting in less significant data on evaluating valve replacement. These factors should be taken into consideration in future longer-term follow-up studies.
In summary, this study demonstrated satisfactory clinical effectiveness of BalMedic bovine pericardial bioprosthetic valves. We will continue the follow-up to obtain more data over a longer term.
Limitations
This is a single-center, retrospective study with potential data and recall biases. While most patients (98.28%) completed the follow-up, a few patients did not, which may have resulted in underestimation of complications. In this study, the maximum follow-up time was 14 years (mean: 7.7±2.5) after BalMedic bioprosthetic valve replacement, which falls in the range of intermediate- to long-term follow-up with respect to statistical significance. Longer-term follow-up is needed to obtain additional data. We did not propose any hypothesis or predefined measures, while this is the first valuable large follow-up study of BalMedic bioprosthetic valves in Chinese patients with valve disease who undergo surgical treatment.
Approved by the CFDA in 2003, BalMedic valves have been used in clinical practice for nearly 17 years. Five surgical centers in China have followed up more than 200 patients for up to 10 years. This follow-up study at our hospital demonstrates good durability and reliable clinical effectiveness of BalMedic valves in Chinese patients with valve disease, with low complication and mortality rates (similar to those of similar foreign counterparts used in developed countries). We will continue to follow up these patients to obtain additional data. Moreover, we will expand this follow-up study to compare the effectiveness of BalMedic valves andmechanical valves implanted in the same time period.
Conclusions
With the first intermediate- to long-term follow-up data of large patients group implantation, bovine pericardial prothetic valve products from Beijing Balane Medical Co. Ltd. demonstrate quite acceptable clinical results compared with its counterparts from foreign manufacturers. In AVR group results, the statistics even shows better outcomes in the rate of survival, freedom from reoperation as well as freedom from SVD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3790
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3790
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3790). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Ethics Committee of The First People’s Hospital of Yulin. The tissue valves implanted are all products obtained premarket approval from CFDA (China Food and Drug Administration).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sidhu P, Okane H, Ali N, et al. Mechanical or bioprosthetic valves in the elderly: a 20-year comparison. Ann Thorac Surg 2001;71:S257-60. [Crossref] [PubMed]
- Antoniou A, Harky A, Yap J, et al. Bioprosthetic aortic valve replacement: a telltale from the young. Ann Transl Med 2018;6:185. [Crossref] [PubMed]
- Singh M, Sporn ZA, Schaff HV, et al. ACC/AHA Versus ESC Guidelines on Prosthetic Heart Valve Management: JACC Guideline Comparison. J Am Coll Cardiol 2019;73:1707-18. [Crossref] [PubMed]
- Han QQ, Xu ZY, Zhang BR, et al. Primary triple valve surgery for advanced rheumatic heart disease in Mainland China: a single-center experience with 871 clinical cases. Eur J Cardiothorac Surg 2007;31:845-50. [Crossref] [PubMed]
- Wang Y, Chen S, Hu XJ, et al. Mid- to Long-term Clinical Outcomes of Hancock II Bioprosthesis in Chinese Population. Chin Med J (Engl) 2015;128:3317-23. [Crossref] [PubMed]
- Barnett SD, Ad N. Surgery for aortic and mitral valve disease in the United States: a trend of change in surgical practice between 1998 and 2005. J Thorac Cardiovasc Surg 2009;137:1422-9. [Crossref] [PubMed]
- Vyavahare N, Ogle M, Schoen FJ, et al. Mechanisms of bioprosthetic heart valve failure: Fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res 1999;46:44-50. [Crossref] [PubMed]
- Cunanan CM, Cabiling CM, Dinh TT, et al. Tissue characterization and calcification potential of commercial bioprosthetic heart valves. Ann Thorac Surg 2001;71:S417-S421. [Crossref] [PubMed]
- Jin L, Zhu XD, Song LF, et al. Experimental study I of the effect of hydroxyl chromium in attenuating calcification. The anticalcification effect of a hydroxyl chromium–modified bovine pericardial test patch in a rat subcutaneous implant model. Chinese Journal of Thoracic and Cardiovascular Surgery 1996:176-8.
- Jin L, Zhu XD, Zhu JM, et al. Experimental study III of the effect of hydroxyl chromium in attenuating calcification. The anticalcification effect of a hydroxyl chromium–modified bovine pericardial test patch in a canine circulatory system implant model. Chinese Journal of Thoracic and Cardiovascular Surgery 1996;(5):62-4.
- Mykén PS, Bech-Hansen O. A 20-year experience of 1712 patients with the Biocor porcine bioprosthesis. J Thorac Cardiovasc Surg 2009;137:76-81. [Crossref] [PubMed]
- Bourguignon T, Bouquiaux-Stablo AL, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg 2014;148:2004-2011.e1. [Crossref] [PubMed]
- Bourguignon T, Espitalier F, Pantaleon C, et al. Bioprosthetic mitral valve replacement in patient aged 65years or younger: long-term outcomes with the Carpentier-Edwards PERIMOUNT pericardial valve. Eur J Cardiothorac Surg 2018;54:302-9. [Crossref] [PubMed]
- Guo H, Lu C, Huang H, et al. Long-Term Clinical Outcomes of the Carpentier-Edwards Perimount Pericardial Bioprosthesis in Chinese Patients with Single or Multiple Valve Replacement in Aortic, Mitral, or Tricuspid Positions. Cardiology 2017;138:97-106. [Crossref] [PubMed]
- Poirer NC, Pelletier LC, Pellerin M, et al. 15-Year Experience With the Carpentier-Edwards Pericardial Bioprosthesis. Ann Thorac Surg 1998;66:S57-61. [Crossref] [PubMed]
- Liu XY, Liu YH. Research progress on factors of early bioprosthesis degeneration. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2015:1060-3.
- Jamieson WR, Burr LH, Miyagishima RT, et al. Carpentier-Edwards supra-annular aortic porcine bioprosthesis: clinical performance over 20 years. J Thorac Cardiovasc Surg 2005;130:994-1000. [Crossref] [PubMed]
- Borger MA, Ivanov J, Armstrong S, et al. Twenty-year results of the Hancock II bioprosthesis. J Heart Valve Dis 2006;15:49-55; discussion 55-6. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the manage. Circulation 2006;114:84-231. [Crossref]