
Intraoperative venous air embolism in the non-cardiac surgery-the role of perioperative echocardiography in a case series report
Introduction
Venous air embolism (VAE) is commonly iatrogenic due to infusion of bubbles adhering to the intravenous (IV) infusion set, residual air in drug-filled syrings, or ambient air intruding into the venous system in diverse surgical procedures (1). In previous reports, this incidence of VAE in neurosurgery conducted in the sitting position varies with a wide range from 4.9% to 76% (2,3). The prevalence of this adverse event during the laparoscopic surgery is less than 0.6%, while as high as 10–50% in hysteroscopic endometrial ablation procedure (4,5). The severity of VAE and its hazards to patients depend not only on the solubility of certain gas in blood, the rate and amount of gas entering the vein, but also the ability to eliminate the air via the lungs (6). Massive air or gas (3–5 mL/kg) entrained in the main pulmonary artery trunk, owing to the air-lock effect, results in acute right ventricle outflow tract (RVOT) obstruction and severe hypoxia, which is a leading cause contributory to cardiac arrest in operation room (1). In some scenarios, VAE was refractory to the routine ACLS (advanced cardiovascular life support intervention), requiring extracorporeal circulation support to achieve the return of spontaneous circulation (7).
Herein, we reported this case serials of VAE in recent 6 years in our department. Albeit VAE has the features of sudden onset, rapid development, and intractable management, the mortality or morbidity remains relatively low if standardized management are strictly implemented in the operation theatre including scrutinizing the patients prior to high-risk surgery, vigilance to the early signs of VAE, timely ceasing the surgical manipulation, as well as meticulous titration of vasoactive agents under the guidance of echocardiography. We present the following case series in accordance with the GARE reporting checklist.
Case presentation
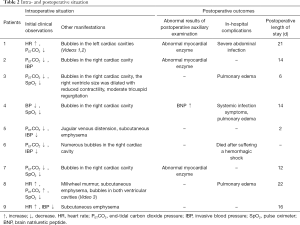
The demographic characteristics and surgical data of all patients are listed in Table 1. Once the patients arriving at the operating room, the IV access was established. The routine monitors including electrocardiogram (ECG), heart rate (HR), blood pressure (BP), pulse oximeter (SpO2), end-tidal carbon dioxide pressure (PETCO2) were applied before general anesthesia induction. The invasive BP (IBP) monitor was established via left radial arterial catheterization, which is also facilitated in blood gas sample collection. Except femoral vein catheterization in case 6, the deep venous catheters were placed in the right internal jugular vein for monitoring central venous pressure (CVP) monitoring in other eight patients. Anesthesia was induced with dose of midazolam (0.05 mg/kg), 1% propofol (1–2 mg/kg), sufentanil (0.25–0.5 µg/kg) and vecuronium (0.1 mg/kg). Endotracheal tube was intubated and secured at the incisors 21–23 cm from tip of the tube. The ventilator was set to volume-controlled ventilation mode (tidal volume at 8–12 mL/kg, respiratory rate at 12 times/min, inspiration and expiration ratio at 1:2) after confirming that the endotracheal tube was in place. The PETCO2 was maintained between 35 and 45 mmHg via adjusting the tidal volume. Anesthesia was maintained with 2% propofol (50–100 µg/kg/min), remifentanil (0.1 µg/kg/min), cisatracurium (2 µg/kg/min), and dexmedetomidine (0.1–0.2 µg/kg/h). Table 2 summarized the additional information about intraoperative conditions.

Full table
Full table
In the case 1 undergoing an open surgery of resection of gallbladder cancer, we noticed the patient promptly developed tachycardia over 120 bpm with decrease of end-tidal CO2 when the surgeons were manipulating the bleeding of hepatic vein minor approximate to retrohepatic inferior vena cava (RIVC). We promptly performed the transesophageal echocardiography (TEE) scanning within 2 minutes after onset of end-tidal CO2 drop. TEE explicitly showed air bubbles in left side of heart chambers (Videos 1,2). Unexpectedly, we couldn’t detect any air in the right-side heart in this patient with paradoxical air embolism all time. The patient did have the patent foramen ovale (PFO) identified by intraoperative TEE and postoperative agitated saline test with transthoracic echocardiography (TTE), and there existed the prerequisite of intracardiac defects contributory to paradoxical air embolism. However, the source of venous air of this was still undetermined yet. The bleed from hepatic vein was tackled with anastomosis without any difficulty and the surgical field was soaked with saline gauzes. Meanwhile, the patient was placed on the Trendelenburg position. The air lodged in systemic circulation lasted approximately 30 mins with no obvious hemodynamic parameters fluctuation and deterioration in oxygenation. TEE examination showed that there is no evident regional wall motion abnormality of left ventricle (LV) or global LV decompensation secondary to coronary artery air embolism. Therefore, the surgery was resumed and accomplished uneventfully. The patient, fortunately, had a smooth recovery with no sign of ischemia stroke complicated with systemic air embolism. In the case 8 undergoing laparoscopic hepatectomy, when the surgeons were manipulating the right hepatic vein approximating second hepatic hilar (portal visceral), the patient was manifested by tachycardia with gradual decrease in SpO2 to 96% on 100% oxygen ventilation. The surgical intervention was suspended and pneumoperitoneum was discontinued instantly after the confirmation of the mill-wheel murmur on the left chest. Bed-side ultrasound showed numerous bubbles in both ventricular cavities on Apical Four-Chambers View (Video 3). The preoperative cardiac ultrasonography did not show any significant intracardiac right-to-left shunt and there is no evidence of pulmonary arteriovenous fistula from chest computer-tomography (CT) in this case. The vital signs of the patient returned to normal following the rapid infusion of Lactated Ringer’s solution concomitant with norepinephrine infusion at 0.05 µg/kg/min. The patient recovered uneventfully without any sign of neurologic deficit from general anesthesia.
The patient 4 undergoing robot-assisted prostate resection, developed severe hypotension and hypoxia when position of the patient returned to supine from deep Trendelenburg position at the end of surgery. The hypotension was refractory to the conventional fluid infusion and vasoconstrictor treatment for 30 min. The point of care ultrasound was used to the assess the cause of the circulatory decompensation. The size of right ventricle moderately dilated with slight shift of intraventricular septum towards the LV. The quantitative analysis of the right ventricle systolic pressure via the peak flow velocity of tricuspid valve regurgitation and tricuspid annular plane systolic excursion (TAPSE) with M modality indicated that the right ventricle contractility was moderately depressed due to elevated pulmonary arterial pressure. The TTE also showed that the well-filled LV squeezed quite well without regional wall motion abnormality. Therefore, the infusion of norepinephrine started at 0.1 µg/kg/min and the BP gradually improved. The patient was subject to moderate pulmonary infiltration in bilateral lower lobes on post-operation of day (POD) 2–3. The patient 8 also had pulmonary edema leading to moderate hypoxia after surgery. The pathogenesis of gas embolism-induced pulmonary edema is complex. Air bubbles-induced turbulent flow activates platelet which leads to the release of inflammatory cytokine or chemokine, recruitment of neutrophils resulting in a series of inflammatory reactions to pulmonary arteriole endothelium (8). The interstitial pulmonary edema was completed dissipated under the PEEP treatment. The recovery of these two patients were uneventful thereafter.
Other postoperative informations were described in Table 2.
Discussion
VAE is a rare but potentially catastrophic complication in non-cardiac surgery. In this 6-year case series, there are 7 cases eventually diagnosed with VAE with echocardiography, two “presumptive” cases incapability to be proved with echocardiography due to poor image quality. It has been proved that the incidence of VAE varies according to the type of medical interventions or surgical operation. Consistent with the previous reports (3,9,10), the hepatic surgery (2/9), neurosurgery (1/9), as well as laparoscopic surgery (5/9) remains the high-risk procedure contributing to VAE in this study. The prognostic outcome of these patients was encouraging, and all patients were discharged uneventfully from hospital without any neurologic sequelae except one neurosurgical case who died of postoperative hemorrhagic stroke complicated from cerebral vascular anastomosis per se.
Diagnosis of VAE
The tachycardia with ST-T segment changes is commonly prominent manifestation in patients with mild to moderate volume of air embolism. However, this sign lacks of high specificity during general anesthesia. Generally, the case with abrupt drop in PETCO2 and pulse saturation concomitant with the exaggerated difference between PaCO2 and PETCO2 over 10 mmHg (normal range of 4–5 mmHg) are highly presumptive of VAE. In line with the previous reports (11-13), the early signs of VAE cases in this series were tachycardia and eminent drop in PETCO2. In the laparoscopic procedure, although the value of PETCO2 is not deceased to such an extent as lower as in other procedures due to carbonate dioxide pneumoperitoneum, the gradient between PaCO2 and PETCO2 remains widened (>10 mmHg). With the extensive use of ultrasound technique in the operation theatres recently, it offers a rapid, precise, and real-time approach in detecting VAE (14). TEE has the advantage over transthoracic echo because it provides a high-quality image to delineate the pathophysiological changes of the patient (15). TEE, therefore, has been a promising tool to aid in diagnosis of perioperative VAE, especially in evaluation of the coexisting intracardiac defect, the cause of circulatory collapse as well as improving the prognostic outcome of patients with VAE. The transthoracic echo, as an alternative monitoring modality, is relatively noninvasive, less time-consuming and atraumatic to patient with broader indications compared with TEE. In this study, 6 out of 9 cases were diagnosed with TTE, only one with TEE. We can obtain the images without difficulty from Apical Four-Chamber View to make the diagnosis of VAE with superficial ultrasound except that two patients (2/9) complicated with subcutaneous emphysema.
Paradoxical VAE
According to the criteria of Johnson (16), paradoxical VAE must be considered “proved” when the embolus was found lodged in the abnormal communication between the systemic arterial and venous system, while the presumptive case is considered: (I) embolus in systemic venous circulation, (II) abnormal communication between left and right circulation, (III) arterial system embolization with no evidence of the source of embolus in the left side of heart. In this 6-year case series, there were two cases presumptive to be paradoxical VAE, accounting for 22% (2/9) of all cases with VAE. These two cases, albeit the bubbles were explicitly detected in the left heart chambers, are not conformed to the criteria of the “proved” case for we didn’t see any air traveling through the intracardiac or any transpulmonary communications. Although the case 1 complicated with tiny PFO, the only chambers we can detect the air are the left atrium and LV in an open surgery. We still couldn’t explain the source of these air in the systemic circulation without any evidence of air in the right heart. According to Aboyans’s study (17), only 16 out of 40 cases were identified to have venous and arterial system embolus simultaneously in pulmonary embolism cases, whereas 7% of cases having arterial system embolism solitarily. In case 8, although we can detect the air bubbles in left and right ventricles simultaneously, we still couldn’t uncover the communication between left and right heart. A meta-analysis conducted in 2014 (18) indicated that TEE had a sensitivity of 89.2% and a specificity of 91.4% in detection of PFO. It is difficult to differentiate PFO from pulmonary arteriovenous malformation (PAVM) and the accepted echocardiographic criteria relies on a delay of 3 to 8 cardiac cycles or 2 to 5 s of agitated saline bubbles to appear in the left atrium. Nonetheless, whether the time of bubble appearance can be used as a basis for distinguishing the location of the shunt point is still controversial (19,20). Despite no detectable intracardiac defect existing in some circumstances, pulmonary embolism frequently resulted in the increased right heart pressures that set the stage for right-to-left shunting via a functional PFO, which might account for the occurrence of paradoxical VAE in patients with intact intra-atrial or ventricular septum in the percutaneous nephrolithotomy (PCNL) or hepatectomy surgeries (21,22). The actual passage leading to paradoxical VAE lacks supportive evidence and is still in debate in those patients.
One of the most common complications associated with paradoxical air embolism is postoperative stroke secondary to cerebral vascular embolization. The predominant clinical presentations are paralysis, altered mental status, and coma (23). Hyperbaric oxygen therapy (HBOT) leads to the reduction in the volume of bubbles, aids removal of nitrogen, and improves the oxygenation of potentially hypoxic tissue (24). The air embolization in coronary arteries, its definitive diagnosis based on fluoroscopy or Coronary Arterial angiography, is relatively rare but life-threatening if extensive coronary circulation involved (25). The TTE or TEE, as the frontline screening tool in the operation theatre, can be used to assess the involved coronary artery manifestation of the regional wall motion abnormality via Four-Chamber and LV Short Axis View at mid-papillary muscle plane. The deterioration in hemodynamic conditions after coronary air embolism should be stabilized with inotropic support, coronary vasodilators, and an intra-aortic balloon pump. Cardiopulmonary resuscitation may be necessary in the presence of ventricular arrhythmias or severe LV dysfunction Fortunately, the two cases in this study didn’t develop coronary arterial air embolism leading to acute LV decompensation, which was identified under direct real-time view via echocardiography.
Treatment of VAE
Prevention is far superior to the treatment of VAE. The hazards of high pneumoperitoneum pressure (>14 cmH2O), severe hypovolemic status, steep reverse Trendelenburg position, should be highlighted. The routine management of perioperative VAE comprises of abrupt cessation of surgical manipulation and pneumoperitoneum, the surgical field soaked with saline solution. All patient susceptible to air embolism were placed at Trendelenburg position immediately following suspending the surgical procedure in this study. The cessation of air-oxygen mixture was implemented and high FiO2 is mandatory in this scenario (26,27).
The circulatory collapse complicated with VAE is not uncommon. There are 3 cases in this series (3/9) subject to refractory hypotension, which was ultimately tackled with inotropic support by norepinephrine and dobutamine infusion under the guidance of echocardiography. In patient with sluggish circulation after air embolism, the right heart function plays a pivotal role in maintaining the stability of systemic circulation. The goal of adjustment of hemodynamic parameters is enhancing the contractility of right heart and ensuring the adequate right coronary artery blood perfusion by increasing the systemic vascular resistance. TTE provides invaluable information of LV myocardial contractility, valvular stenosis or insufficiency, the volume status, right heart function as well as the exclusion of the obstructive factors comprising of tamponade, pleural effusion, or pneumothorax. In case 3, the right ventricle size was dilated with reduced contractility and moderate tricuspid regurgitation indicating elevated pulmonary arterial pressure posterior to air lock in RVOT. The commencement of norepinephrine infusion contributed to reversal of circulatory depression.
Conclusions
In addition to improving the detection of VAE, echocardiography has a pivotal role in assessment of the right ventricle dysfunction in massive PE and guiding the pharmacotherapy in refractory hypotension after VAE (28). The presumptive case of paradoxical air embolism is not infrequent to encounter if routine monitor with the echocardiography is implemented in this case-series. The assessment of coronary artery occlusion and regional wall motion abnormality is of paramount importance post air embolism. Therefore, point-of-care ultrasound is not only facilitated in the diagnosis of VAE but also tailoring the treatment of VAE complicated the severe cardiovascular decompensation.
Acknowledgments
Funding: This research was supported by the Nanjing Science and Technology Development Foundation, No. QRX17013; the Six Talent Summit Project of Jiangsu Province, No. WSN-147; the Nanjing Health Commission of Nanjing Municipal Government, No. YKK17084.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-Wei Tu) for the series “Hemodynamic monitoring in critically ill patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-497). The series “Hemodynamic monitoring in critically ill patients” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gordy S, Rowell S. Vascular air embolism. Int J Crit Illn Inj Sci 2013;3:73-6. [Crossref] [PubMed]
- Maragkos GA, Davanzo J, Roberts SM, et al. Paradoxical air embolism without patent foramen ovale during craniotomy in the sitting position. Cureus 2019;11:e4355. [PubMed]
- Ammirati M, Lamki TT, Shaw AB, et al. A streamlined protocol for the use of the semi-sitting position in neurosurgery: a report on 48 consecutive procedures. J Clin Neurosci 2013;20:32-4. [Crossref] [PubMed]
- Neudecker J, Sauerland S, Neugebauer E, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc 2002;16:1121-43. [Crossref] [PubMed]
- Groenman FA, Peters LW, Rademaker BM, et al. Embolism of air and gas in hysteroscopic procedures: pathophysiology and implication for daily practice. J Minim Invasive Gynecol 2008;15:241-7. [Crossref] [PubMed]
- Liang Y, Rice MJ. Venous air embolism: the severity depends on many factors. Anesth Analg 2017;124:1733-4. [Crossref] [PubMed]
- Panchal AR, Berg KM, Hirsch KG, et al. 2019 American Heart Association Focused Update on Advanced Cardiovascular Life Support: Use of Advanced Airways, Vasopressors, and Extracorporeal Cardiopulmonary Resuscitation During Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2019;140:e881-94. [PubMed]
- Peng CK, Huang KL, Wu CP, et al. Phosphodiesterase-4 Inhibitor Roflumilast Attenuates Pulmonary Air Emboli-Induced Lung Injury. J Surg Res 2019;241:24-30. [Crossref] [PubMed]
- Poznanska G, Grzelak P, Durczynski A, et al. Venous air embolism during major liver surgery: Far more common, than we think. Eur J Anaesthesiol 2014;31:120-1. [Crossref] [PubMed]
- Sood J. Advancing frontiers in anaesthesiology with laparoscopy. World J Gastroenterol 2014;20:14308-14. [Crossref] [PubMed]
- Mirski MA, Lele AV, Fitzsimmons L, et al. Diagnosis and treatment of vascular air embolism. Anesthesiology 2007;106:164-77. [Crossref] [PubMed]
- Rademaker BM, van Kesteren PJ, de Haan P, et al. How safe is the intravasation limit in hysteroscopic surgery? J Minim Invasive Gynecol 2011;18:355-61. [Crossref] [PubMed]
- Kapurch CJ, Abcejo AS, Pasternak JJ. The relationship between end-expired carbon dioxide tension and severity of venous air embolism during sitting neurosurgical procedures - A contemporary analysis. J Clin Anesth 2018;51:49-54. [Crossref] [PubMed]
- Alrayashi W, Miller T, Vo D. Point-of-care Ultrasound Detection of Intraoperative Venous Air Embolism. Anesthesiology 2017;127:711. [Crossref] [PubMed]
- Starczewska MH, Stach O, Kański A. Will transoesophageal echocardiography become a standard tool for anesthetists to assess haemodynamic status during non-cardiac surgeries? Case report and literature review. J Ultrason 2014;14:435-41. [Crossref] [PubMed]
- Johnson BI. Paradoxical embolism. J Clin Pathol 1951;4:316-32. [Crossref] [PubMed]
- Aboyans V, Lacroix P, Ostyn E, et al. Diagnosis and management of entrapped embolus through a patent foramen ovale. Eur J Cardiothorac Surg 1998;14:624-8. [Crossref] [PubMed]
- Mojadidi MK, Bogush N, Caceres JD, et al. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: a meta-analysis. Echocardiography 2014;31:752-8. [Crossref] [PubMed]
- Frazin LJ. Patent foramen ovale or pulmonary arteriovenous malformation: an appeal for diagnostic accuracy. Chest 2007;132:5-6. [Crossref] [PubMed]
- Zukotynski K, Chan R, Chow C, et al. Contrast echocardiography grading predicts pulmonary arteriovenous malformations on CT. Chest 2007;132:18-23. [Crossref] [PubMed]
- Kawahara T, Hagiwara M, Takahashi H, et al. Cerebral Infarction by Paradoxical Gas Embolism During Laparoscopic Liver Resection with Injury of the Hepatic Vessels in a Patient without a Right-to-Left Systemic Shunt. Am J Case Rep 2017;18:687-91. [Crossref] [PubMed]
- Chahal D, Ruzhynsky V, McAuley I, et al. Paradoxical air embolism during percutaneous nephrolithotomy due to patent foramen ovale: Case report. Can Urol Assoc J 2015;9:E658-60. [Crossref] [PubMed]
- Scruggs JE, Joffe A, Wood KE. Paradoxical air embolism successfully treated with hyperbaric oxygen. J Intensive Care Med 2008;23:204-9. [Crossref] [PubMed]
- Edsell ME, Kirk-Bayley J. Hyperbaric oxygen therapy for arterial gas embolism. Br J Anaesth 2009;103:306. [Crossref] [PubMed]
- Clayton DG, Evans P, Williams C, et al. Paradoxical air embolism during neurosurgery. Anaesthesia 1985;40:981-9. [Crossref] [PubMed]
- Law AD, Gulati A, Bhalla A. Air in the heart: what should one do? Am J Emerg Med 2012;30:1659.e1-3.
- Verma A, Singh MP. Venous gas embolism in operative hysteroscopy: a devastating complication in a relatively simple surgery. J Anaesthesiol Clin Pharmacol 2018;34:103-6. [PubMed]
- Miranda-Bacallado J, Izquierdo-Gómez MM, García-Niebla J, et al. Role of echocardiography in a patient with suspected acute pulmonary embolism: a case report. J Med Case Rep 2019;13:37. [Crossref] [PubMed]


