
When is the optimal timing of frozen embryo transfer after controlled ovarian stimulation?
The frozen-thawed embryo transfer (FET) in the process of assisted reproductive technologies (ART) has been increasingly performed in the last few decades (1). The development of controlled ovarian stimulation (COS) protocol has flourished accordingly with the increase of surplus embryos and embryo freezing technique, and the freeze-all policy has been established to overcome potential adverse carryover effects of COS (1).
COS is an important preliminary phase of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) to obtain the highest oocyte retrieval possible, but its underlying supraphysiological hormonal status might disrupt the synchronization of endometrial receptivity and embryo maturity, the essential component in implantation success. Also, even when the endometrium is prepared and subsequent fresh embryo transfer is performed, the risk of ovarian hyperstimulation syndrome should still be recognized (2,3) Thus, despite of potentially disputable aspects, numerous physicians have adopted the freeze-all policy after COS and FET to minimized such unfavorable conditions (4).
The other general consensus is that patients are avoided to proceed to FET in their subsequent menstrual cycle after freezing embryo; the theoretical background of FET deferral is that the negative effect on endometrial receptivity caused by COS could be continued until next menstrual cycle (5-7). Thus, according to recent clinical trials, FET deferral for at least on menstrual cycle has been widely accepted by many international scholars (8-11).
However, some scholars argue that the residual effect of COS on endometrial receptivity on the next cycle is mostly based on speculation; in addition, delayed FET and inevitable passage of time could possibly lay unnecessary emotional stress to patients and even lead to drop-out from infertility treatment (12). Therefore, there is a need for reconsideration of the timing of FET after COS based on evidence-based approach.
Huang et al., in their latest retrospective cohort study of FET timing in non-elective freeze-all cycles, compare reproductive outcomes in the two groups: one group with FET immediately within the first menstrual cycle after COS and the other group with delayed FET to subsequent cycles (12). They conclude that the rates of implantation, clinical pregnancy, ongoing pregnancy and live birth were all reduced in the group with delayed FET.
In order to evaluate the clinical outcomes of immediate or delayed FET, in-depth review considering multiple parameters is necessary. Table 1 shows the list of comprehensive studies regarding timing of FET after COS. As stated in Table 1, freeze-all and fresh-failed cycle need be analyzed separately when comparing the timing of FET. In freeze-all cycle, the synchrony of stage of embryo and endometrial growth is given less priority by clinicians; on the other hand, in fresh cycle, COS has been carried out with caution for this synchrony. Moreover, fresh-failed cycle suggests that the medications for luteal support have been used and that such medications may have influence on subsequent cycle.
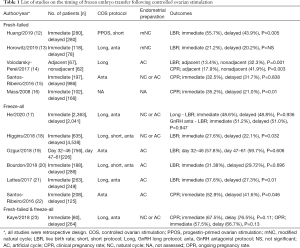
Full table
Fresh-failed cycles
In 4 studies with fresh-failed cycle, immediate FET showed no difference or better live birth rate than delayed FET (12,13,15,16). However, the study of Volodarsky-Perel et al., which included only GnRH agonist long protocol, reported contrasting results; the hormonal profile and function of corpus luteum after oocyte pick-up was quite different between GnRH agonist long protocol and GnRH antagonist protocol (14). In GnRH agonist long protocol, GnRH receptors were downregulated and recovery of GnRH receptors took longer periods than GnRH antagonist protocol. Also, hCG used in GnRH agonist long protocol had longer half-life and could have had impact on corpus luteal function and endometrial receptivity of subsequent cycle. Based on published literatures, in fresh-failed cycle, immediate FET was assumed to have better or at least no harm compared with delayed FET except GnRH long protocol.
However, several limitations still exist. In the study of Huang et al., as the authors pointed out, the number of patients included in the study were not enough to reach statistical significance (12). In the study of Horowitz et al., they suggested the possibility of practical bias (13).
Freeze-all cycles
Regarding freeze-all cycles, most studies have showed the immediate FET had benefit or no difference on clinical outcomes in compared to delayed FET (17-22). Recently, He et al. reported the outcomes of FET according to timing after COS in combination with the analysis of each COS protocol separately, GnRH agonist long protocol and GnRH antagonist protocol (17). In this study, there was no significant difference in the rates of live birth, implantation, clinical pregnancy, multiple pregnancy, early miscarriage, premature birth and stillbirth between immediate and delayed FET groups in the same COS protocol (17).
Suggested mechanisms for immediate or delay FET
Adverse effects of high endogenous hormonal value in COS cycle were suggested as the reasons of applying delayed FET (12,13). The abnormal hormonal levels might cause the altered hypothalamic-pituitary-ovarian (HPO) axis, dysfunctional corpora lutea and the altered endometrial receptivity (12). The altered HPO axis was evident by delayed ovulation in the subsequent natural cycle following COS (13). Nevertheless, in the study of Huang et al., the live birth rate was not different in both groups, and the authors stated that the delay of ovulation day had no influence on the clinical outcomes. Yet again, as mentioned in their study, the cohort had insufficient power to detect clinically significance (12).
Regarding dysfunctional corpora lutea, Huang et al. indicated that abrupt luteolysis after GnRH agonist triggering had no influence in the outcomes of subsequent FET cycle because the type of ovulation triggering was not related to pregnancy outcome in FET cycles (12). However, luteal support protocol is quite different between GnRH agonist triggering and hCG triggering. In GnRH agonist triggering cycle, many clinicians use low dose hCG as luteal support method. These aspects are not fully ruled out as a related factor in subsequent FET cycle.
As an implantation promoting factor in immediate FET, higher serum level of relaxin produced by multiple corpus luteum was suggested by Huang et al. (12). However, it is not obvious that the multiple corpus luteum could produce higher serum level of relaxin (24). Also, even if serum level of relaxin could be higher in COS cycle, it is unclear that it could be continued at subsequent menstrual cycle.
Conclusion
The latest findings of Huang et al. suggest that there is no benefit in delaying FET for subsequent menstrual cycles; such approach could help patients to reduce associated emotional stress and anxiety throughout ART process (12). However, all the studies are retrospective design and the possibility of publication, selection and practical biases are existed. Currently, the first randomized controlled trial (RCT) comparing successful pregnancy rate of immediate versus delayed FET after COS is ongoing; the study includes 724 patients, 362 with immediate FET and 362 with delayed FET, estimated to be completed in June, 2020 (25). Successful publication of such study would provide interesting and valuable information on controversies regarding the optimal timing of FET after COS.
Acknowledgments
Funding: None
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.41). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roque M, Valle M, Kostolias A, et al. Freeze-all cycle in reproductive medicine: current perspectives. JBRA Assist Reprod 2017;21:49-53. [Crossref] [PubMed]
- Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod 2011;26:2593-7. [Crossref] [PubMed]
- Roque M, Lattes K, Serra S, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril 2013;99:156-62. [Crossref] [PubMed]
- Blockeel C, Drakopoulos P, Santos-Ribeiro S, et al. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod 2016;31:491-7. [Crossref] [PubMed]
- Develioglu OH, Hsiu JG, Nikas G, et al. Endometrial estrogen and progesterone receptor and pinopode expression in stimulated cycles of oocyte donors. Fertil Steril 1999;71:1040-7. [Crossref] [PubMed]
- Shapiro BS, Daneshmand ST, Garner FC, et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011;96:344-8. [Crossref] [PubMed]
- Zapantis G, Szmyga MJ, Rybak EA, et al. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod 2013;28:3292-300. [Crossref] [PubMed]
- Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523-33. [Crossref] [PubMed]
- Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126-36. [Crossref] [PubMed]
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393:1310-8. [Crossref] [PubMed]
- Weissman A. IVF worldwide survey results: frozen-thawed embryo transfer. 2017. Available online: https://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryo-transfer.html
- Huang J, Lu X, Xie Q, et al. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Transl Med 2019;7:752. [Crossref] [PubMed]
- Horowitz E, Mizrachi Y, Farhi J, et al. Modified natural cycle cryopreserved embryo transfer: is a washout period needed after a failed fresh cycle? Reprod Biomed Online 2019;39:439-45. [Crossref] [PubMed]
- Volodarsky-Perel A, Eldar-Geva T, Holzer HE, et al. Cryopreserved embryo transfer: adjacent or non-adjacent to failed fresh long GnRH-agonist protocol IVF cycle. Reprod Biomed Online 2017;34:267-73. [Crossref] [PubMed]
- Santos-Ribeiro S, Siffain J, Polyzos NP, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril 2016;105:1202-7.e1. [Crossref] [PubMed]
- Maas K, Baker V, Westphal L, et al. Optimal timing of frozen embryo transfer after failed IVF attempt. Fertil Steril 2008;90:S285. [Crossref]
- He Y, Zheng H, Du H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol 2020;18:1. [Crossref] [PubMed]
- Higgins C, Healey M, Jatkar S, et al. Interval between IVF stimulation cycle and frozen embryo transfer: Is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol 2018;58:217-21. [Crossref] [PubMed]
- Ozgur K, Bulut H, Berkkanoglu M, et al. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet 2018;35:135-42. [Crossref] [PubMed]
- Bourdon M, Santulli P, Maignien C, et al. The interval between oocyte retrieval and frozen-thawed blastocyst transfer does not affect the live birth rate and obstetrical outcomes. PLoS One 2018;13:e0206067. [Crossref] [PubMed]
- Lattes K, Checa M, Vassena R, et al. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod 2017;32:368-74. [Crossref] [PubMed]
- Santos-Ribeiro S, Polyzos NP, Lan VTN, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod 2016;31:2541-8. [Crossref] [PubMed]
- Kaye L, Marsidi A, Rai P, et al. Frozen blastocyst transfer outcomes in immediate versus delayed subsequent cycles following GnRH agonist or hCG triggers. J Assist Reprod Genet 2018;35:669-75. [Crossref] [PubMed]
- Arthur ID, Anthony FW, Adams S, et al. Serum relaxin and the major endometrial secretory proteins in in-vitro fertilization and down-regulated hormone-supported and natural cycle frozen embryo transfer. Hum Reprod 1996;11:88-91. [Crossref] [PubMed]
- Li H, Li L, Lu X, et al. Comparison of the effect of immediate versus delayed transfer following a stimulated IVF cycle on the ongoing pregnancy rate of frozen-thawed embryo transfer cycles: a study protocol for a randomised controlled trial. BMJ Open 2018;8:e020507. [Crossref] [PubMed]


