
Ultrasonographic appearance of triple-negative invasive breast carcinoma is associated with novel molecular subtypes based on transcriptomic analysis
Introduction
Triple-negative breast cancer (TNBC) is recognized as an aggressive disease with poor prognosis among breast cancers (1,2). The lack of an effective target therapy for estrogen receptor (ER), progesterone receptor (PR), or human epidermal receptor 2 (HER2) further worsen the prognosis of TNBCs (3). TNBC is also well known as a heterogeneous disease with various biological properties of tumors and clinical outcomes (4-7). Based on cytokeratins (4), transcriptomes (6), or genomics (7-9), TNBCs are divided to four molecular subgroups (4,6,7) or six subtypes (9) that are associated with the clinical outcome of TNBCs.
Ultrasonography plays an important role in the screening of breast cancers, particularly in Asian populations. Similar to the biological property of the tumors, TNBCs show a considerable variety in ultrasonographic features (10-14). The heterogeneity of sonographic features of TNBCs in particular of the benign-like sonographic appearance in some TNBC patients, hinders early and accurate diagnosis. We have previously demonstrated that the benign-like TNBCs are more proliferative (15) than those with typical malignant sonographic features. Therefore, missing TNBC lesions in the ultrasound (US) may cause serious consequences. The genetic origin of sonographic variety is critical for the accurate recognition of specified molecular subtypes with poor prognosis.
In the present study, we aimed to evaluate the association between sonographic features and four molecular subtypes based on the integrated expression profiles of both mRNA and long non-coding RNA (lncRNA) (6). The effects of demographics, tumor-node-metastasis (TNM) stage, sonographic features, pathological features, and transcriptome-based molecular subtypes on the clinical outcome defined as recurrence-free survival (RFS) and overall survival (OS) were also studied.
Methods
Patient recruitment
This was a retrospective observational study on the prospectively collected data between 1 January 2010 and 31 December 2013. A total of 114 consecutive patients who accepted surgical treatment for unilateral breast cancers without ER, PR, and HER2 at Fudan University Shanghai Cancer Center (FUSCC) were included. The examination results for chest computed tomography (CT), bone scan, abdominal US, bilateral mammography, breast US, and/or magnetic resonance imaging (MRI) were collected to ascertain no metastasis beyond breasts and axillary lymph nodes occurred before the surgery.
Sample preparation, microarray experiment, and clusters
Breast tumor samples were well preserved in liquid nitrogen for further processing. The tumor area was macro-dissected to ensure tumor cells accounted for 90% or more of the whole breast cancer specimen. Total RNA expression was detected from the frozen TNBC samples using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA). NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used for checking the purity and quantity of total RNA by measuring absorbance at 260 and 280 nm with RNase-free water as a blank control. RNAs with the ratio of A260/A280 within 1.9–2.1 qualified for further experiments. The transcriptomic profiles of the breast tumor samples were determined using GeneChip Human Transcriptome Array 2.0 (HTA 2.0) (Affymetrix, Santa Clara, CA, USA) with details described in our earlier publications (5-7).
Four stable clusters, named immunomodulatory (IM), luminal androgen receptor (LAR) mesenchymal-like (MES), and basal-like and immune-suppressed (BLIS) were identified after analyzing the robustness of the classification using k-means clustering by resampling randomly selected tumor profiles (1,000 iterations) with the details available in our previous study (6). Furthermore, gene oncology (GO) and pathway analyses were performed to identify the top GO and canonical pathways associated with the four TNBC subtypes as demonstrated in Table 1. The detailed activation and inhibition patterns were comprehensively illustrated previously (6). Our classification system, named FUSCC, correlated well with the Lehmann/Pietenpol classification system (6,7).

Full table
Clinical and pathological data
The ER, PR, and HER2 status of the breast tumor samples was confirmed by two experienced pathologists (WTY and RHS) based on immunohistochemical analysis and in situ hybridization. TNBC was defined as ER, PR, HER2 negative in accordance with the St. Gallen International Expert Consensus (16). The clinical, pathological, and immunohistochemical characteristics were collected including age, menopausal status, tumor size, tumor histological grade, lymph node status, Ki67 level, and adjuvant therapies. Patients were categorized according to age (<50 and ≥50 yrs), tumor size (≤2 and >2 cm), histological grade (grade I, II, III), and Ki67 level (<40% and ≥40%).
Assessment of ultrasonographic features
US images of the TNBC mass were collected from the data backup server. A second review was conducted by two US physicians who specialized in breast US examination and Breast Imaging Reporting and Data System (BI-RADS) lexicon. The assessment for US images was based on BI-RADS published in 2013 (17). The ultrasonographic features included orientation (parallel and not parallel), shape (regular and irregular), margin (circumscribed and uncircumscribed), angular or spiculated margin (yes and no), echo pattern (hypoechoic, mixed solid echo, complex cystic, and solid echo), posterior acoustic pattern (shadow, enhancement, no change, and mixed pattern), calcification (yes and no) (15,17). During the assessment, the two examiners were mutually blinded to each other as well as the patients’ clinical and pathological data. A consensus was reached for disagreements between the two examiners.
Irregular shape, with angular or spiculated margin, posterior acoustic shadow, and calcification were deemed as typical malignant sonographic signs. Patients were divided into three groups according to the following sonographic features: Group 1, no malignant sonographic features; Group 2, one or two malignant sonographic features; Group 3, three or four malignant sonographic features. Bilateral axillary US examination was performed for each patient.
Patient follow-up
Follow-up of all patients in the cohort was completed on 31 August 2018. The median length of follow-up was 63 months with the interquartile range of 53–70 months. The RFS events were defined as follows: the first recurrence of invasive breast cancer at a local, regional, or distant site; the incidence of contralateral breast cancer, and death from any causes. Patients without RFS events were censored at the last follow-up. All data included in the present study was collected after obtaining ethical approval from the institutional review board at FUSCC (No. 1802181-22-NSFC). Informed written consent was obtained from all patients.
Statistical analysis
SPSS for Windows version 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Student’s t-test was used to compare continuous variables, and Pearson’s chi-square test was used for comparing categorical data. Multivariate Cox regression analysis was used to determine the variables associated with RFS and OS. Adjusted hazard ratio (HR) and the 95% confidence interval (CI) were calculated with Cox proportional-hazards model. Survival curves for both RFS and OS were plotted using the Kaplan-Meier method and used to compare BLIS with the other subtypes using the log rank test.
Results
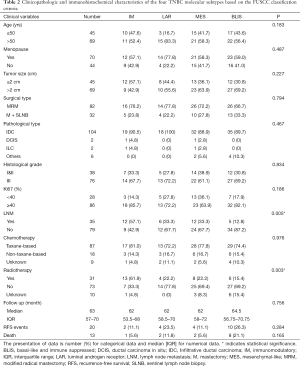
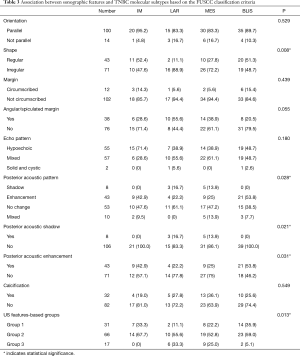
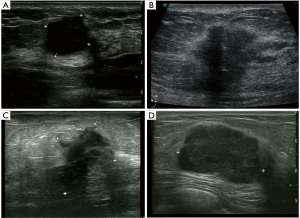
According to the expression of mRNA and lncRNA, 114 patients were classified into the following four molecular subtypes: 21 cases (18.4%) in IM group, 18 cases (15.8%) in LAR group, 36 cases (31.6%) in MES group, and 39 cases (34.2%) in BLIS group. Clinical, pathological, and ultrasonographic data were presented according to the four subtypes as demonstrated in Table 2 and Table 3. BLIS subtype showed the lowest incidence of axillary lymph node metastasis and the lowest rate of radiotherapy compared to the other three subtypes (P<0.05). IM subtype showed the highest incidence of axillary lymph node metastasis and the highest rate of radiotherapy compared to the other three subtypes (P<0.05). There was no significant difference among the four subtypes in terms of patient age, tumor size, surgery type, pathological grade, Ki67 level, chemotherapy, follow-up duration, and incidence of RFS or death events (P>0.05). With respect to ultrasonographic features, the four molecular subtypes showed significant differences in terms of tumor shape (P=0.008), posterior acoustic pattern (P=0.028), and sonographic appearance-based subgroups (P=0.013) as shown in Table 3. Compared with LAR and MES subgroups, IM and BLIS subgroups had higher probability of presenting benign-like sonographic features, e.g., regular shape, no angular/spiculated margin, and posterior acoustic enhancement (P<0.05). Figure 1 shows the typical sonographic images for TNBC masses of the four molecular subtypes.
Full table
Full table
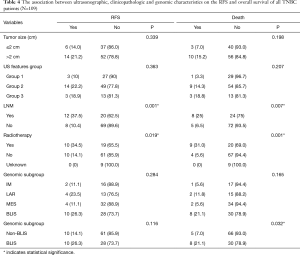
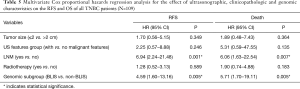
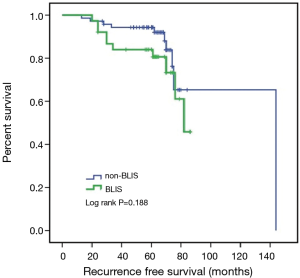
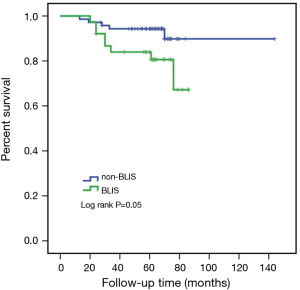
Due to the cessation of follow-up within four years after the surgery, 109 patients were included in the survival-related analysis after excluding five patients. The univariate Logistic regression analysis shows that post-operative LNM and radiotherapy treatment were significantly associated with RFS and death events (P<0.05, Table 4). Meanwhile, BLIS subtype was significantly associated with the death event (P<0.05, Table 4). Multivariate Cox regression analysis demonstrated that independent risk factors for RFS and death events of TNBCs included the presence of postoperative axillary lymph node metastasis (P<0.05) and the BLIS subtype (P<0.05) as shown in Table 5. Figures 2 and 3 show the Kaplan-Meier survival curves for RFS and OS. BLIS subtype presented significantly worse OS than other TNBC subtypes (log rank P=0.05), while there was no significant difference in RFS (log rank P=0.188).
Full table
Full table
Discussion
Summary of main findings
TNBCs are associated with high heterogeneity, aggressive proliferation, and low differentiation (1,18,19). Currently, there are limited effective target therapies catering to the heterogeneity of TNBCs (20,21). The heterogeneity of TNBCs has been observed in US imaging features as well as at cellular and genetic levels. However, there is a lack of studies on the association between sonographic features and genetic properties. In the present study, we classified 114 TNBC patients into four subtypes according to the expression profiles of both mRNAs and lncRNAs. The classification system for TNBC named FUSCC was established and verified by our research team. The sonographic features in terms of tumor shape and posterior acoustic pattern were found to be significantly associated with the four molecular subtypes of TNBC. IM and BLIS subtypes showed similar sonographic appearance with higher probability to present benign-like sonographic features than LAR and MES groups. However, sonographic features were not found to be significantly associated with the RFS and OS of TNBC. The survival outcome of TNBC is significantly associated with the axillary lymph node metastasis and the molecular subtypes. BLIS subtype showed the worst prognosis compared with the other four subtypes.
Validated and multi-omics profiling based classification system
The establishment of our classification system FUSCC was based on integrating the expression profiles of both mRNAs and lncRNAs from 165 TNBC specimens (6) and was verified with genomic analysis (7). Compared to the Lehmann/Pietenpol classification that includes six molecular subtypes according to the integrated analysis of 14 publicly available RNA profiles (9), the FUSCC classification system is more simplified. In FUSCC, the BLIS subtype included the BL1 and BL2 subtypes in the Lehmann/Pietenpol classification. It was also concordant with BLIS subtype showing the worst survival outcome compared to the same subtype in previous studies (8). In the subsequent genomic analysis, the FUSCC classification system was further validated and subdivided for the search of more potential targeted therapeutic biomarkers within specific subtypes (7). For example, the genomic data suggested that east Asian TNBCs tended to present enriched ERBB2 (Erb-B2 receptor tyrosine kinase 2) mutation in the LAR subtype. BLIS TNBC can be further classified depending on low or high homologous recombination deficiency (HRD) score. TNBC patients with high HRD scores tended to have a favorable prognosis and may benefit from DNA-damaging chemotherapy or DNA repair inhibitors (7). These findings may imply the necessity for the adjustment of clinical management for east Asian TNBCs (7).
Heterogeneity of TNBC sonographic features
As reported in previous studies, the most common sonographic features for TNBCs included regular shape, no angular/spiculated margin, posterior acoustic enhancement, and no calcifications which are characteristic for benign breast masses (10,11,13,22,23). However, not all TNBC masses present these features. In the present study, the incidence was 37.7% for regular tumor shape, 66.7% for no angular/spiculated margin, 37.7% for posterior acoustic enhancement, and 71.9% for no calcification. Zhang et al., in their study of 1,000 cases, reported that TNBCs had only 28.7% probability of having circumscribed margins and 42.5% probability of having a regular shape (24). The imaging heterogeneity has been an obstacle in the early and accurate diagnosis of TNBCs, in particular for those breast masses with benign-like sonographic features in young patients (15).
Clinical implication of the association between biological heterogeneity and sonographic variety
Information obtained from medical imaging has been proven to be closely related to the characteristics of genes, proteins, and tumor phenotypes (25-27). In our study, we also found that sonographic features in terms of tumor shape and posterior acoustic pattern were found to be significantly associated with the four molecular subtypes of TNBC. Tumors with active and rapid growth had less matrix interaction with the surrounding breast tissues, which leads to relatively circumscribed margins (28,29). The composition of enriched tumor cells, fibrosis tissue, and necrosis may affect the echotexture and posterior acoustic pattern of the breast tumors. It has been reported that TNBCs with infiltrative tumor border pattern was associated with luminal cluster and poor prognosis and pushing border pattern was associated with basal cluster and good prognosis (4). Their findings indicated that benign-like TNBCs might have better prognosis than those with malignant-like appearance. This is consistent with our finding that TNBCs with no malignant or one or two malignant sonographic features tended to show better prognosis than those with at least three malignant sonographic features. However, it is difficult to explain the finding that BLIS subtype that had the highest probability to show benign sonographic appearance had the worst prognosis. We postulate that the qualitative assessment of sonographic features based on BI-RADS lexicon was not sufficient to explain the controversial clinical outcome between IM and BLIS subtypes that had similar sonographic features. Future research using high-throughput images analysis to quantify sonographic features is warranted to evaluate the association between radiomics and genomics of TNBCs. The modified FUSCC classification system based on genomic analysis can be used to better differentiate TNBC masses with similar sonographic features.
The other question about BLIS subtype is related to the association between axillary lymph node metastasis and clinical outcome. BLIS subtype showed the lowest incidence of axillary lymph node metastasis. IM subtype showed the highest incidence of axillary lymph node metastasis. While, surprisingly, BLIS TNBCs had worse prognosis than IM subtype TNBCs. This means that the effect of postoperative axillary lymph nodes on the clinical outcome should be interpreted after considering the FUSCC classification system for TNBCs.
Nowadays, individualized treatment of TNBCs mainly focuses on the biomolecular characteristics detected by genomics and proteomics (30). The FUSCC classification system based on the transcriptomic landscape can identify different molecular subtypes of TNBCs and demonstrate the heterogeneity of biological properties (5,7). Now at our institute, clinical trials about personalized treatment based on the genomic analysis are undertaking for some refractory TNBC cases. We expect that this classification will benefit TNBC patients in terms of precision treatment and will be used worldwide in the future. However, examination of genomics in routine clinical practice is challenging due to the complicated process and high expense. Sonographic images are always acquired prior to clinical, pathological, and genomic characteristics. Future studies will focus on evaluating associations between sonographic radiomics and genomics and exploring the clinical significance of sonographic features for treatment decision-making and prognosis prediction.
Limitations
The following limitations should be considered in the present study: The US images were retrospectively retrieved from the image backup system dating back to 5–7 years ago. The unsatisfactory image quality in addition to the objective assessment for still US images may lead to misinterpretation of the sonographic features. The sample size was too small to predict the clinical outcome using sonographic features, although there was a trend in our results that TNBCs with benign features had more favorable prognosis than those with malignant features.
Conclusions
Although ultrasonographic features of TNBCs did not contribute to the survival outcome significantly, sonographic appearance is associated with the transcriptome-based molecular subtypes that are associated with prognosis and survival. The sonographic features can be valuable for predicting the transcriptomic expression of TNBCs.
Acknowledgments
We appreciate Dr. Jing Yuan from the Department of Clinical Statistics in Fudan University Shanghai Cancer Center for her help in offering the information on patient’s follow-up. Great gratitude should be given to Dr. Fei Liang for his specialized consultation regarding the statistical analysis for survival curves.
Funding: This study got foundation supports from the National Natural Science Foundation of China (No. 81627804 and 81830058).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.204). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All data included in the present study was collected after obtaining ethical approval from the institutional review board at Fudan University Shanghai Cancer Center (No. 1802181-22-NSFC). Informed written consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [Crossref] [PubMed]
- Di Micco R, Santurro L, Gasparri ML, et al. Rare sites of breast cancer metastasis: a review. Transl Cancer Res 2019;8:S518-52. [Crossref]
- Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA 2018;319:154-64. [Crossref] [PubMed]
- Elsawaf Z, Sinn HP, Rom J, et al. Biological subtypes of triple-negative breast cancer are associated with distinct morphological changes and clinical behaviour. Breast 2013;22:986-92. [Crossref] [PubMed]
- Jiang YZ, Liu YR, Xu XE, et al. Transcriptome analysis of triple-negative breast cancer reveals an integrated mRNA-lncRNA signature with predictive and prognostic value. Cancer Res 2016;76:2105-14. [Crossref] [PubMed]
- Liu YR, Jiang YZ, Xu XE, et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res 2016;18:33. [Crossref] [PubMed]
- Jiang YZ, Ma D, Suo C, et al. Genomic and transcriptomic landscape of Triple-Negative Breast Cancers: subtypes and treatment strategies. Cancer Cell 2019;35:428-40 e5.
- Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688-98. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Yang Q, Liu HY, Liu D, et al. Ultrasonographic features of triple-negative breast cancer: a comparison with other breast cancer subtypes. Asian Pac J Cancer Prev 2015;16:3229-32. [Crossref] [PubMed]
- Sannomiya N, Hattori Y, Ueda N, et al. Correlation between ultrasound findings of tumor margin and clinicopathological findings in patients with invasive ductal carcinoma of the breast. Yonago Acta Med 2016;59:163-8. [PubMed]
- Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology 2009;250:638-47. [Crossref] [PubMed]
- Boisserie-Lacroix M, Macgrogan G, Debled M, et al. Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist 2013;18:802-11. [Crossref] [PubMed]
- Wojcinski S, Stefanidou N, Hillemanns P, et al. The biology of malignant breast tumors has an impact on the presentation in ultrasound: an analysis of 315 cases. BMC Womens Health 2013;13:47. [Crossref] [PubMed]
- Li JW, Zhang K, Shi ZT, et al. Triple-negative invasive breast carcinoma: the association between the sonographic appearances with clinicopathological feature. Sci Rep 2018;8:9040. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Mendelson EB, Böhm-Vélez M, Berg WA. ACR BI-RADS® Ultrasound. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013.
- Li CY, Zhang S, Zhang XB, et al. Clinicopathological and prognostic characteristics of triple- negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev 2013;14:3779-84. [Crossref] [PubMed]
- Liao HY, Zhang WW, Sun JY, et al. The clinicopathological features and survival outcomes of different histological subtypes in triple-negative breast cancer. J Cancer 2018;9:296-303. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674-90. [Crossref] [PubMed]
- Celebi F, Pilanci KN, Ordu C, et al. The role of ultrasonographic findings to predict molecular subtype, histologic grade, and hormone receptor status of breast cancer. Diagn Interv Radiol 2015;21:448-53. [Crossref] [PubMed]
- Zheng FY, Lu Q, Huang BJ, et al. Imaging features of automated breast volume scanner: Correlation with molecular subtypes of breast cancer. Eur J Radiol 2017;86:267-75. [Crossref] [PubMed]
- Zhang L, Li J, Xiao Y, et al. Identifying ultrasound and clinical features of breast cancer molecular subtypes by ensemble decision. Sci Rep 2015;5:11085. [Crossref] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278:563-77. [Crossref] [PubMed]
- Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. [Crossref] [PubMed]
- Wen X, Yu Y, Yu X, et al. Correlations Between Ultrasonographic Findings of Invasive Lobular Carcinoma of the Breast and Intrinsic Subtypes. Ultraschall Med 2019;40:764-70. [Crossref] [PubMed]
- Costantini M, Belli P, Bufi E, et al. Association between sonographic appearances of breast cancers and their histopathologic features and biomarkers. J Clin Ultrasound 2016;44:26-33. [Crossref] [PubMed]
- Tamaki K, Ishida T, Miyashita M, et al. Correlation between mammographic findings and corresponding histopathology: potential predictors for biological characteristics of breast diseases. Cancer Sci 2011;102:2179-85. [Crossref] [PubMed]
- Shen M, Jiang YZ, Wei Y, et al. Tinagl1 Suppresses Triple-Negative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling. Cancer Cell 2019;35:64-80.e7. [Crossref] [PubMed]


