
An algorithm for one-stage malignant oncologic scalp reconstruction
Introduction
The scalp is the thickest skin in the human body, covering the pericardium and not only playing an essential role in appearance but also further protecting the intracranial structures. Scalp carcinomas derive from the skin and can invade adjacent, particularly deep calvarium structures. As the most effective treatment, surgery undoubtedly results in the scalp defect. There are a substantial number of modern surgical techniques, including primary closure, local flap, skin graft, pedicle flap, and free flap, which help to reconstruct surgical defect satisfactorily. Nevertheless, the reconstruction of the scalp still is challenging because of the convexity of the underlying skull and the fixing of scalp layers, which leads to a small defect hard to close (1-3).
Meanwhile, the defect following radical resection of the malignancy is characterized by irregular shape and exposure of the inner structures. One-stage reconstruction can result in the ideal functional and aesthetic outcome and avoid intractable complications, such as delayed wound healing, infection, and cerebrospinal fluid leakage. However, the reconstructive procedures are based on a negative surgical margin bringing challenges to preoperative planning. Although various literatures reported clear reconstructive guidelines, there are very scarce systematic data about scalp carcinomas. The majority of published literature recommended the types of reconstructions were based on the attempt to obtain negative surgical margins; it is scarce of the systemic guidelines for various scalp defects following radical surgical resections.
Our retrospective study aims to extract the findings from our experience and develops a reconstructive algorithm of one-stage scalp reconstruction for scalp malignancies.
Methods
We retrospectively reviewed patients with diagnoses of scalp malignancies and underwent curative attempted surgeries from November 2002 to February 2018 at the Head and Neck Surgery Department, Si Chuan Cancer Hospital. Patients who could not tolerate surgery or had distant metastatic disease were excluded. Preoperative head CT and/or MRI were used to assess the tumor’s extension.. All tumors histopathology specimens and resection margins status were recorded. The operative spectrum for scalp reconstruction included primary closure, local flap, skin graft, pedicle flap, and free flap.
After referring to the classification of 3 references about scalp defect (4-6), We reclassified defect sizes as small (≤4 cm2), defect (4–30 cm2), large (30–90 cm2), extra-large (>90 cm2) and total scalp defect. The defect size was equal to the long axis multiple the longest axis perpendicular to the long axis. Defect depth was pathologic measurements defined as partial-thickness, full-thickness of the scalp, and calvarium or/and dura defect. Tumor nature was defined as primary or recurrent disease. Data of tumor nature, scalp defect location, size, and depth, and reconstructive procedures were collected and analyzed retrospectively. An algorithm consisted of five reconstruction techniques according to various disease and surgical defect scenarios. The technologies of adjacent tissue expanding, vacuum therapy, and integra dermal regeneration template were not included in the algorithm because those were considered as a two-step procedure.
Results
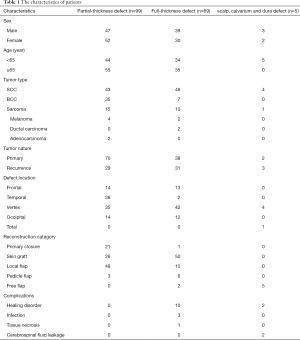
A total of 173 patients were included. Eighty-nine patients were males, and 84 were females. The age ranged from 11 to 96, with a mean age of 62.1 years old. Histopathology consisted with squamous cell carcinoma (n=95, 54.9%), basal cell carcinoma (n=42, 24.3%), sarcoma (n=26, 15.0%), melanoma (n=6, 3.5%), adenocarcinoma (n=2, 1.2%) and ductal carcinoma (n=2, 1.2%). There were no intraoperative complications or surgery associated with mortality. Thirteen patients developed postoperative wound complications (7.5%). Eight patients developed a single complication, and the rest of the 5 patients had complex complications. The characteristics of patients and are presented in Table 1. The distribution of scalp defect size and depth and the relation between scalp defect and reconstructive methods is the list in Tables 2 and 3, respectively.
Full table
Full table

Full table
Primary closure
A total of 22 cases underwent primary closure of the defects. The average defect size was 3.9 cm2 (range, 2.0 to 9.0 cm2). Twenty of these defects were partial-thickness, and 1 was full-thickness of the scalp. The primary closure was the most straightforward technique with short operating time, and less likely to cause surgical complications. It would bring the best cosmetic and cost-effectiveness results at the same time.
Nevertheless, with the increase of defect size, the primary closure procedure becomes more challenging. The limit of mobilization of residual scalp makes the wound more in tension. For a sizeable surgical defect, there is an increased risk of wound dehiscence and local tissue ischemia, no matter the depth of the surgical defect. Although there are subtle differences in the loose and tight scalp when we perform the reconstruction, the tension-free closure can be achieved through a proper dissection and loosen the scalp. So, the defect size plays a vital role in this surgical technique rather than defect depth and location.
Local flap
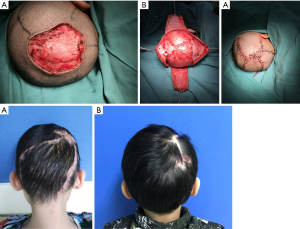
A total of 59 reconstructions were performed with local flaps. The average defect size was 15.0 cm2 (range, 4.0 to 56.0 cm2). Forty-nine of defects were partial-thickness, and 10 were full-thickness. Our experience showed that rotated local scalp tissue is the best technique to close the defect. If we could not close the surgical defect directly, the local flaps would be the next consideration. Advanced flap (Figure 1) and rotated flap (Figure 2) were the main surgical techniques. In the loose area of the scalp, it was easy to close these defects by either advanced or rotated flaps, even with a deep defect to calvarium or dura.
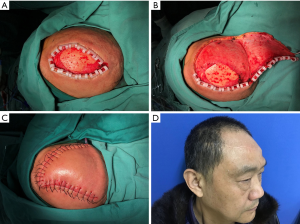
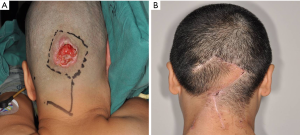
Nevertheless, in the tight scalp area, the advanced flap reconstruction could not work well owing to the inelastic nature even if the defect were small. Instead, we favored to rotated flap reconstruction or combination of advanced and rotated flaps both. Three patients displayed surgical complications due to the tension of wound closure. One of them was wound healing disorder, and the other two were wound infection. All these healed by dressing change and wound care.
Skin graft
A total of 76 reconstructions were performed with skin grafts. The average defect size was 37.0 cm2 (range, 4.0 to 168.0 cm2). Twenty-six of defects were partial-thickness, and 50 were full-thickness. The procedure of skin grafts was as easy as primary closure.
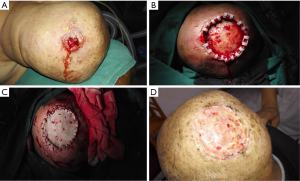
Furthermore, it could cover the full size of surgical defects. However, harvesting a skin would create a new tissue defect, cosmetic and functional outcomes are often considerably less satisfactory. The blood supply in the scalp tissue is the key to skin graft survival. When the defects are full-thickness, we would burr and drilling small holes of the outer table of the calvaria (Figure 3) in order to create a better blood supply to the skin graft. There were no significant complications saw except only 1 massive skin graft necrosis, 1 wound infection, and 4 other types of wound healing problems.
Pedicle flap
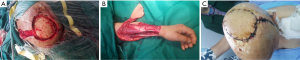
There was 9 pedicle flap reconstruction performed in the cohort. The average defect size in pedicle flap reconstruction was 56.9 cm2 (range, 30 to 95 cm2). Three of the defects were partial-thickness, and 6 were full-thickness. The pedicle flap reconstructions included temporoparietal fascia flap, trapezius flap (Figure 4), and the latissimus dorsi musculocutaneous flap.
Free flap
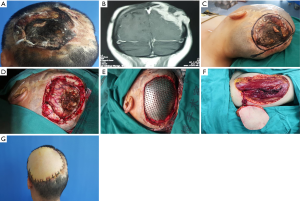
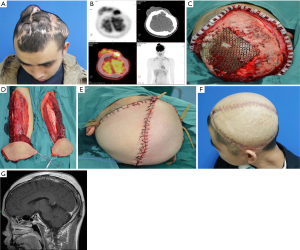
There were 7 cases reconstructed with free flaps. The defects ranged from 90 to 400 cm2, consisting of 1 significant defect, 5 extra-large defects, and 1 total scalp defect. The defects of 2 cases were full-thickness, and the others included the defect of scalp, calvarium, and dura. Two cases were reconstructed with radial forearm flap (Figure 5) and anterolateral thigh flap in 6 cases (Figures 6,7). The average size of a single flap was 124.0 cm2 (range, 90 to 200 cm2). Vascular supplies in recipient and donor sites were evaluated preoperatively by Doppler ultrasound. The superficial temporal artery and vein were anastomosed as the recipient’s vessel. Two patients suffered from the defect of scalp, calvarium, and dura underwent the recipient site complications of wound healing disorder and cerebrospinal fluid leakage. Proper intracranial pressure control and careful wound care are the keys to reduce potential postoperative complications. There were no significant complications in the donor site. All donor sites were primarily closed except two cases with split-thickness skin grafting in the radial forearm donor sites.
Algorithm
An algorithm based on defect size, depth, location, and five reconstructive methods was set up (Figures 8-10).
Discussion
Radical resection is the most fundamental curative way, improving prognosis and lay the foundation for adjuvant therapies. We have matured reconstructive skills and advanced materials, but the knowledge about reconstructive options is limited, and the evaluation of the tumor and physical condition is insufficient.
We can generally found from our findings (Tables 2 and 3) that the smaller the defects were, the few layers of the scalp the tumors invaded. And with the growth of tumors, the defects were more complex, including not only the size but also the depth and even some inner structures destroyed. The simplest methods matched simple defects, mostly for functional and cosmetic goals. The complex defects should take account for complex reconstructive procedures for functional purposes primarily, and some cases were only suitable for simple methods for lower complications risks.
The algorithm for primary malignant oncologic scalp reconstruction
Primary closure should always be considered first for the small defects for its cosmetic outcome and minimal healing time. If the strain of skin exists, slight subgaleal dissection is necessary. When the strain is intense in some full-thickness defects, or primary closure still cannot achieve with considerable undermining, we usually give priority to local flaps. Local flaps are routinely used to repair medium defects as either advancement flaps, transporting flaps, or rotational flaps. Even if more substantial amounts of undermining must achieve flaps mobility, the donor site can provide the best match in thickness, color, texture, and sensation. However, the procedure of local flaps must consider the histopathology of the tumor, possible defect area and the laxity of adjacent tissues. If there are some difficulties for young or unexperienced doctors in flap planning, they can choose skin grafts, with smooth conducting and fewer complications. When the defect is more than 30 cm2, single local flap can rarely succeed. If we insist use a local flap, larger or multiple ones must be used, and anatomy and vascular territories should evaluate preoperatively. Even if well preparation has been done, the tension and extensive undermining of flaps undoubtfully increase the risk of infection, healing delay and wound dehiscence. What is worse, the attention should be strengthened on vascular trunk entering the scalp, avoiding when some patients need to perform the lateral dissection. We do not recommend local flaps for the significant defect unless the aesthetic preference. A skin graft is a constructive method of quick, easy, and safe, supplying coverage to defects directly without other elements. This makes it an outstanding possibility for the reconstruction of more substantial defects after oncologic and nononcologic resection.
However, some authors hold the opinion that this reconstructive method usually results in a suboptimal cosmetic outcome and may not be as durable as autologous skin (7). Practically, we find the grafting in parietal and temporal parts can be shaded by the adjacent hair and which locate in occipital part can also be covered by long hair, especially in women’s. For some patients bald or barely hair, the skin will not have any influence on appearance. However, some worries that skin graft can only survive if pericranium or galea exists after radical resection (8). The cranial bone itself does not own a vascular bed to provide enough blood supply. Some manipulations such as drilling, chiseling, milling, burring, or carbon dioxide laser help remove portions of the outer table to promoting granulation tissue formatting for the survival of grafting skin (9-11). And the risk of dura or intradural structures injury is minimal with these manipulation techniques (12).
In terms of the blood supply in significant full-thickness defects, pedicle flaps with flexible structure and robust vascular supply near defects could rotate to cover the cranial bone. Temporoparietal fascia flap, based on the branches of the superficial temporal artery, is a versatile method to repair defects in frontal, parietal, and temporal areas, but the use of this method must combine with a split-thickness skin graft to protect deep layers (13-15). And because of its limited length of the pedicle, distal occipital defects can choose lower-region trapezius flap and the latissimus dorsi musculocutaneous flap (16,17). Theoretically, trapezius and latissimus dorsi flaps have a very long-dominant vascular pedicle which generally can reach any region of the scalp, and does not need skin graft as temporoparietal fascia flap dose (8,16). However, to reconstruct the frontal, temporal, and vertex defects, overstretched pedicle and gravity owing to the transfer of flap location decrease blood flow volume and velocity, leading to ischemia at the distal part of the flap quickly. And the pedicle flap itself leads to one more area defect and cause increased morbidity. Thus, the pedicle flap is a practical alternative for significant scalp defects. Among them, temporoparietal fascia flap is suitable for the full-thickness defect in frontal, vertex and temporal areas, and trapezius flap and latissimus dorsi musculocutaneous flap can usually be chosen for both part and full-thickness defects in the posterior scalp.
When the defect is more than 90 cm2, part necrosis of grafting skin will often happen if it is used. Furthermore, the abundant mount harvest of the skin increases the complications of donor sites at the same time. A more size and safe method should be considered. Free flaps have the bulk of vascularized tissues that can be designed to match the defects nicely. The microsurgical vessel anastomosis guarantee flap safety and recovery after surgery. Popular flaps options include anterolateral thigh flap, radial forearm flap, latissimus dorsi flap, omental flap, rectus abdominis flap, and scapular flap (18-21). Our overall flap success rate was 100%, with the mean size of 124.0 cm2 exceeding 90 cm2 easily, supplying satisfied outcomes. However, it should be noted that the redundant subcutaneous tissues may result in poor scalp shape, and this is also the main factor influencing healing. There are some factors such as elder age, short surgical preparation, inadequate quality of recipient’s vessels, cardiopulmonary diseases, and nonproficiency in microsurgery affect flap choosing and even surviving. Some reconstructions conservative, stable, and uncomplicated should ensure the surgery if patients were unable to tolerate free flap transfer. As we mentioned above, skin graft, popular in head and neck region with broad indications and scarcely restricted by defect size, could be the alternative for extra-large defects, especially in frontal, vertex and temporal areas. However, we do not recommend free flap transfer as the first option for the occipital part unless the pedicle is long enough to anastomose with vessels in tempora or neck. Therefore, the pedicle flap, such as trapezius and latissimus dorsi, still can make full use of their abundant tissues and long pedicles in extra-large defects.
The algorithm for recurrent malignant oncologic scalp reconstruction
The patients who presented to our hospital for recurrent surgery required complex reconstructions. These patients undergone an earlier surgical procedure or radiation therapy bring challenges to reconstruction. This is because significant fibrosis and scarring increase tension, decrease available scalp, and delay healing. The small defect can mostly close directly by large subgaleal undermine.
Nevertheless, with the enlarging of defect size, repeated recurrence should be on alert. We recommend skin graft as the first option instead of a local flap owing to its detecting tumor recurrence earlier than any flap reconstruction without significant function damage (22,23). The use of local flap is restricted by excessive tension and limited adjacent scalp. It is not the mainstream compared with primary treatment.
We can find there is no noticeable difference in the reconstruction choice of extra-large defects between primary and recurrent tumors. Flaps with a large number of tissues and reliable blood supply are the key to ensure the functionality of the scalp.
When the recurrent carcinoma spreads over the scalp, the whole scalp must be resected for oncological radicals. Multiple flaps combinations are reported for the complex reconstruction, including free anterolateral thigh and latissimus dorsi flaps, bilateral free anterolateral thigh flap, serratus anterior, and latissimus dorsi flaps, bilateral latissimus dorsi flap (24-26) to protect the calvarium or even the inner structure. Additionally, some single free flaps also have worked in the total scalp defect. Hussussian et al. used a combination of free latissimus dorsi muscle and split-thickness skin graft for coverage of 900 cm2 (27). Labow et al. used a combination of split-thickness skin graft and free omentum, latissimus dorsi muscle, or rectus abdominous muscle to reconstructive total scalp defect (28). Although we can get enough size from the single free flap, one or multiple split-thickness skin harvest is bound to increase the secondary donor site complications. And the extensive free flap with one pedicle may not supply reliable blood for some cases. Therefore, the complications including infection wound dehiscence, split-thickness skin graft loss, were far more common (28,29). Double free flaps were separated with two vessels and the flaps solve the insufficient blood supply effectively and every flap can harvest at a proper size, decreasing the tension for donor site primary closure.
The algorithm for scalp, calvarium and dura defect reconstruction
The large infiltrating or long-history recurrent tumor often invades not only the scalp but the inner tissues such as calvarium, dura, and brain. Once the oncological resection involves a craniectomy and durotomy, the defects will become more complex. A single-stage reconstruction should consider not only the scalp but also inner structures. Free flaps are useful to reconstruct the intractable due to their abundant tissue and vascularity (30). Dura plays an imperative role in eliminating dead space. For dura reconstruction, synthetic artificial materials, and autologous fascia are widely used (31,32). However, there are some reports about the infection of alloplastic materials (33). Once the intracranial infection happened, the antibiotic therapy alone is inadequate, a second surgery with enough debridement and removal of alien materials is needed. Compared with it, autologous fascia has lower complications rate (deep structures infection and cerebrospinal fluid leak) and cost to surgery and hospital stay (34,35). The fascia lata is the most common autograft. And ALT flap can supply the advantage of customizable size fascia lata for dural defect, which lends preference to the scalp, calvarium, and dura defect (36). Although the defect of the cranial, dura, or even both of them do not influence the choice of the free flap for reconstruction (37), we should be familiar with every common free flap and its harvest procedure for choosing the most suitable one for safety and function.
Conclusions
The oncologic radicality directly affects patient survival, and any reconstruction is based on radical resection. Modern reconstructive categories and technologies allow one-stage of reconstruction of any extent of malignant scalp tumor. However, successful reconstruction depends on a comprehensive understanding of scalp anatomy and adequate preoperative planning. The presented algorithm is intended to guide scalp reconstruction varying in size, depth, and location to offer patients the best outcomes.
Acknowledgments
Funding: This study was supported by the Major Science and Technology Project Foundation of Sichuan Science and Technology Department (2019YFS0337).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.221). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frodel JL Jr, Ahlstrom K. Reconstruction of complex scalp defects: the“Banana Peel” revisited. Arch Facial Plast Surg 2004;6:54-60. [Crossref] [PubMed]
- Gundeslioglu AO, Selimoglu MN, Doldurucu T, et al. Reconstruction of large anterior scalp defects using advancement flaps. J Craniofac Surg 2012;23:1766-9. [Crossref] [PubMed]
- Hoffman JF. Management of scalp defects. Otolaryngol Clin North Am 2001;34:571-82. [Crossref] [PubMed]
- Iblher N, Ziegler MC, Penna V, et al. An algorithm for oncologic scalp reconstruction. Plast Reconstr Surg 2010;126:450-9. [Crossref] [PubMed]
- Vitagliano T, Greco M, Dessy LA, et al. Surgical strategies for cutaneous neoplasm of the scalp. State of art. Ann Ital Chir 2015;86:117-25. [PubMed]
- Desai SC, Sand JP, Sharon JD, et al. Scalp reconstruction: an algorithmic approach and systematic review. JAMA Facial Plast Surg 2015;17:56-66. [Crossref] [PubMed]
- Newman MI, Hanasono MM, Disa JJ, et al. Scalp reconstruction: A 15-year experience. Ann Plast Surg 2004;52:501-6. [Crossref] [PubMed]
- Jeong WS, Roh JL, Kim EK. Extensive scalp reconstruction after repeated failure of free tissue transfer with a pedicled latissimus dorsi flap. J Craniofac Surg 2014;25:1103-5. [Crossref] [PubMed]
- Vanderveen EE, Stoner JG, Swanson NA. Chiseling of exposed bone to stimulate granulation tissue after Mohs surgery. J Dermatol Surg Oncol 1983;9:925-8. [Crossref] [PubMed]
- Bailin PL, Wheeland RG. Carbon dioxide (CO2) laser perforation of exposed cranial bone to stimulate granulation tissue. Plast Reconstr Surg 1985;75:898-902. [Crossref] [PubMed]
- Foong DP, Babar AZ, McGrouther DA, et al. Key points in technique: split thickness skin grafting to bare skull as a single stage procedure. J Plast Reconstr Aesthet Surg 2008;61:1260-3. [Crossref] [PubMed]
- Mühlstädt M, Thome C, Kunte C. Rapid wound healing of scalp wounds devoid of periosteum with milling of the outer table and split-thickness skin grafting. Br J Dermatol 2012;167:343-7. [Crossref] [PubMed]
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg 2001;3:170-7. [Crossref] [PubMed]
- Kurbonov U, Davlatov A, Janobilova S, et al. The Use of Temporoparietal Fascia Flap for Surgical Treatment of Traumatic Auricle Defects. Plast Reconstr Surg Glob Open 2018;6:e1741. [Crossref] [PubMed]
- Ozkan O, Ozkan O, Bektaş G, et al. Reconstruction of a distal extremity defect using a temporoparietal fascia flap covered with a split-thickness skin graft harvested from the scalp: a cosmetic consideration in donor site selection. Ulus Travma Acil Cerrahi Derg 2012;18:207-12. [Crossref] [PubMed]
- Har-El G, Bhaya M, Sundaram K. Latissimus dorsi myocutaneous flap for secondary head and neck reconstruction. Am J Otolaryngol 1999;20:287-93. [Crossref] [PubMed]
- Park SO, Chang H. Discussion: The Trapezius Muscle Flap: A Viable Alternative for Posterior Scalp and Neck. Arch Plast Surg 2016;43:536-7. [Crossref] [PubMed]
- Chang KP, Lai CH, Chang CH, et al. Free flap options for reconstruction of complicated scalp and calvarial defects: report of a series of cases and literature review. Microsurgery 2010;30:13-8. [PubMed]
- Lutz BS, Wei FC, Chen HC, et al. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg 1998;51:186-90. [Crossref] [PubMed]
- Disa JJ, Pusic AL, Hidalgo DH, et al. Simplifying microvascular head and neck reconstruction: a rational approach to donor site selection. Ann Plast Surg 2001;47:385-9. [Crossref] [PubMed]
- Lynch JR, Hansen JE, Chaffoo R, et al. The lower trapezius musculocutaneous flap revisited: versatile coverage for complicated wounds to the posterior cervical and occipital regions based on the deep branch of the transverse cervical artery. Plast Reconstr Surg 2002;109:444-50. [Crossref] [PubMed]
- Corradino B, Di Lorenzo S, Leto Barone AA, et al. Reconstruction of full thickness scalp defects after tumour excision in elderly patients: our experience with Integra dermal regeneration template. J Plast Reconstr Aesthet Surg 2010;63:e245-7. [Crossref] [PubMed]
- Dedhia R, Luu Q. Scalp reconstruction. Curr Opin Otolaryngol Head Neck Surg 2015;23:407-14. [Crossref] [PubMed]
- Kwee MM, Rozen WM, Ting JW, et al. Total scalp reconstruction with bilateral anterolateral thigh flaps. Microsurgery 2012;32:393-6. [Crossref] [PubMed]
- Haddock MC, Creagh T, Sivarajan V. Double-free, flow-through flap reconstruction for complex scalp defects: a case report. Microsurgery 2011;31:327-30. [Crossref] [PubMed]
- Davison SP, Capone AC. Scalp reconstruction with inverted myocutaneous latissimus dorsi free flap and unmeshed skin graft. J Reconstr Microsurg 2011;27:261-6. [Crossref] [PubMed]
- Hussussian CJ, Reece GP. Microsurgical scalp reconstruction in the patient with cancer. Plast Reconstr Surg 2002;109:1828-34. [Crossref] [PubMed]
- Labow BI, Rosen H, Pap SA, et al. Microsurgical reconstruction:A more conservative method of managing large scalp defects? J Reconstr Microsurg 2009;25:465-74. [Crossref] [PubMed]
- McCombe D, Donato R, Hofer SO, et al. Free flaps in the treatment of locally advanced malignancy of the scalp and forehead. Ann Plast Surg 2002;48:600-6. [Crossref] [PubMed]
- Yoshioka N. Versatility of the Latissimus Dorsi Free Flap during the Treatment of Complex Postcraniotomy Surgical Site Infections. Plast Reconstr Surg Glob Open 2017;5:e1355. [Crossref] [PubMed]
- Nakano T, Yoshikawa K, Kunieda T, et al. Treatment for Infection of Artificial Dura Mater Using Free Fascia Lata. J Craniofac Surg 2014;25:1252-5. [Crossref] [PubMed]
- Ozcan U, Akyurek M, Arslan E. Complex Scalp and Calvarium Defects After Giant Basal Cell Carcinoma Excision: Management, Challanges, Outcomes. J Craniofac Surg 2018;29:1273-5. [Crossref] [PubMed]
- Nakagawa S, Hayashi T, Anegawa S, et al. Postoperative infection after duraplasty with expanded polytetrafluoroethylene sheet. Neurol Med Chir (Tokyo) 2003;43:120-4. [Crossref] [PubMed]
- Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery 2004;54:599-603. [Crossref] [PubMed]
- Sabatino G, Della Pepa GM, et al. Autologous dural substitutes: a prospective study. Clin Neurol Neurosurg 2014;116:20-3. [Crossref] [PubMed]
- Lin PY, Miguel R, Chew KY, et al. The role of the anterolateral thigh flap in complex defects of the scalp and cranium. Microsurgery 2014;34:14-9. [Crossref] [PubMed]
- Sweeny L, Eby B, Magnuson JS, et al. Reconstruction of scalp defects with the radial forearm free flap. Head Neck Oncol 2012;4:21. [Crossref] [PubMed]





