
Hemodynamic monitoring in patients with venoarterial extracorporeal membrane oxygenation
IntroductionOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an effective mechanical circulatory support modality that rapidly restores systemic perfusion in cardiogenic shock patients, over days or weeks. Patients receiving VA-ECMO support, and institutions offering ECMO support are rapidly increasing. Generally, the main indications for VA-ECMO are refractory circulatory failures, including medical or post-cardiotomy shock, cardiac arrest, refractory ventricular tachycardia, and the acute management of invasive procedure complications. The fundamental purpose of VA-ECMO support is a bridge to recovery, to heart transplantation, to a more durable bridge, or to decision (1). Currently, there is no consensus on the daily management of VA-ECMO patients, due a lack of clinical evidence. Optimal management approaches involve several inputs such as circulatory support, infection prevention and nutrition support, where hemodynamic monitoring plays a fundamental role in VA-ECMO, from initiation to weaning.
This article provides an overview of VA-ECMO pathophysiology, and reviews current knowledge of hemodynamic monitoring assessments in patients with peripheral VA-ECMO.
VA-ECMO pathophysiologyOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
The basic principles of VA-ECMO
VA-ECMO drains blood from the right atrium using a centrifugal pump, and transits it through an oxygenator, where gas exchange (oxygenation and CO2 removal) occurs. The oxygenated blood returns to the circulation through the large arteries, to maintain systemic perfusion. Notably, during peripheral VA-ECMO support, pulsatile antegrade blood flow ejected by the heart, collides with continuous retrograde perfusion supplied by ECMO, leading to a dynamic mixing cloud in the aorta (a watershed region). The external circulation disrupts normal physiological ventricular-arterial coupling, and impacts cardiac function.
VA-ECMO impact on the heart
The initiation of VA-ECMO markedly decreases right ventricle end-diastolic volume (RVEDV) (2,3). In a fixed right ventricle (RV) contractility and pulmonary vascular resistance setting, decreases in RVEDV lead to reductions in RV stroke volume (Figure 1A). Simultaneously, the returning flow of VA-ECMO elevates systemic mean arterial pressure (MAP), and left ventricle (LV) afterload, as well as maintaining peripheral perfusion. With ECMO flow increases, arterial pressure increases and arterial pulse pressure (PP) decreases and, concomitantly, LV stroke volume decreases (Figure 1B) and the duration of aortic valve opening shortens (4).
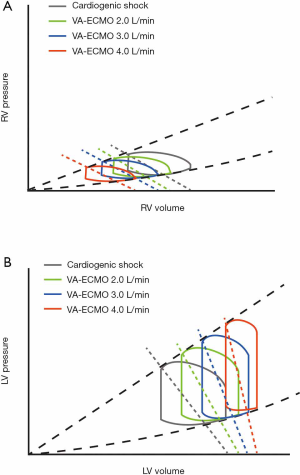
Hemodynamic monitoring in VA-ECMO patientsOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
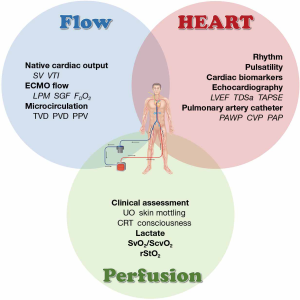
It is important to note that hemodynamic responses during VA-ECMO support are complex, and vary among patients due to multiple clinical variables. We emphasize three dimensions of hemodynamic monitoring: perfusion, flow and cardiac function.
Perfusion assessments
The main role of VA-ECMO support is to provide adequate blood flow and oxygen supply to maintain optimal global tissue perfusion. Several indices exist for the assessment of peripheral perfusion in VA-ECMO patients.
Clinical assessment
Clinical examination provides valuable information on perfusion for patients with VA-ECMO (Figure 2 and Figure 3). Abnormal neurological status (delirium/confusion), cold and clammy skin, and oliguria are common, possible signs of mal-perfusion during VA-ECMO support. The skin allows intensivists to assess peripheral microcirculatory perfusion, using accessible indices such as skin temperature gradients, mottling, and capillary refill time (CRT). Skin mottling is a good predictor of early mortality in septic shock patients (5,6). Skin mottling scoring allows for the increased discrimination of therapy responses (5). CRT is also associated with increased morbidity and mortality, and decreased visceral organ perfusion in critically ill patients (7,8). CRT may be a promising target during the resuscitation of early septic shock, and may be related to lower mortality and rapid improvements in terms of organ dysfunction (9,10). However, these indices may fail to reflect more central tissue perfusion (11), and may be restricted by other circumstances, e.g., dark skin. Until now, no studies have evaluated the potential role of these indices in patients with VA-ECMO.
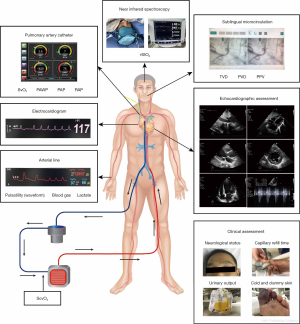
Lactate monitoring
Lactate is a metabolic byproduct of anaerobic glycolysis, and an indicator of inadequate oxygen delivery. It is accepted that elevated serum lactate levels in critically ill patients, is mainly derived from hypoxic origins due to circulatory failure. Hyperlactatemia during the initial phase of shock may reflect inadequate tissue perfusion, and is associated with elevated mortality (12). Elevated serum lactate levels during the early phase of ECMO implantation are associated with increased mortality (13-16). Moreover, lactate clearance rates can also be helpful in monitoring therapy responses. Increasing evidence shows that lactate level changes after ECMO implantation are important prognostic factors (14-17). However, it is important to note that inadequate oxygen delivery or hypoperfusion is not the sole cause of hyperlactatemia. Exogenous catecholamines, stress or impaired liver function can also influence lactate levels (18). Moreover, slow lactate clearance rates often indicate severe microvascular dysfunction.
Mixed venous oxygen saturation (SvO2) and central-venous oxygen saturation (ScvO2)
SvO2 from the pulmonary artery, as an indirect index of tissue oxygenation, is an independent predictor of mortality in septic and cardiogenic shock (19-21). ScvO2 as a surrogate is strongly correlated with SvO2 (22,23). Though ScvO2 as a target for resuscitation of septic shock remains controversial, monitoring ScvO2 levels is still advocated as a simple method in assessing balances between oxygen delivery and consumption, in various clinical settings (24,25). ScvO2 <70% indicates a mismatch between oxygen delivery and consumption (26). Low ScvO2 in early shock stages is associated with mortality in septic shock patients (20,27). For VA-ECMO patients, ECMO circuits provide a platform for real-time, continuous analysis of venous oxygen saturation. Pre-membrane saturation of the ECMO circuit, approximating ScvO2, reflects tissue oxygenation adequacy on VA-ECMO. Low ScvO2 levels are vital warning signs of inadequate oxygen delivery. Several reports have shown that low ScvO2 levels were associated with mortality in VA-ECMO patients (28,29). The causes of decreased ScvO2 are decreased oxygen delivery, or increased extraction. Decreased oxygen delivery results from a low cardiac output (CO) during cardiogenic shock, or severe hypoxia in respiratory failure. Oxygen supplies can be increased by increasing ECMO flow and maintaining adequate MAP, oxygenation, CO and hemoglobin levels. Increased extraction is mainly due to increased metabolic rate or sepsis. Means to decrease the metabolic activity including sedation and hypothermia may be needed.
Regional saturation of tissue oxygen (rStO2)
Patients undergoing ECMO have potentially devastating and often debilitating neurological complications, including ischemic/hemorrhagic stroke or seizures, which are associated with longer hospital stays and increased mortality (30-33). As patients are usually sedated during VA-ECMO support, traditional clinical neurological examinations are not always feasible. Cerebral near-infrared spectroscopy (NIRS) is a non-invasive method that continuously monitors regional saturation of cerebral oxygen (rScO2). It signals the balance between cerebral oxygen delivery, and cerebral oxygen consumption. Many factors affect the accuracy of rScO2 measurements, such as arterial pressure, CO2 concentrations, ECMO blood flow, arterial oxygen saturation, hematocrit, anesthesia levels and regional temperatures. Cerebral desaturation or large right–left rScO2 differences are independently associated with neurologic injury in patients undergoing VA-ECMO (34,35). NIRS can be useful in monitoring lower extremity perfusion in patients receiving VA-ECMO. Lower rStO2 in the cannulated leg or large differences in rStO2 between the legs may indicate lower extremity ischemia (36,37). In conclusion, continuous monitoring of cerebral and lower extremity regional saturation oxygen levels could provide intensivists with early identification and intervention capabilities before the development of irreversible injury, and could serve as an adjunctive indicator of neurologic or lower extremity status in VA-ECMO patients.
Ensuring adequate blood flow
Cardiogenic shock patients are characterized by end-organ hypoperfusion caused by low native CO and hypotension. As a rescue therapy, VA-ECMO provides hemodynamic and gas exchange support, and rapidly restore systemic hemodynamics. Adequate blood flow is the prerequisite to maintaining tissue perfusion. Since VA-ECMO introduces an external circulation coupled to a native cardiopulmonary circulation, functional blood flow is composed of native CO and ECMO flow during ECMO support.
Monitoring VA-ECMO blood flow
The optimal level of ECMO support varies depending on native cardiac function. Patients with severely impaired native cardiac function usually require maximal ECMO support. Levels of ECMO support are determined by the amount of flow the ECMO circuit provided. The initial ECMO flow should be 50–70 mL/kg/min, along with a mean arterial pressure >60 mmHg (38). ECMO flow is modulated to maintain or restore normal hepatic, renal, pulmonary and neurological functions.
ECMO flow is influenced by the modifiable variables of preload, afterload, and impellor revolutions per minute (RPM), as well as by static variables of diameter and cannula length. In general, increased RPM could directly increase blood flow. In a fixed RPM setting, a drop in ECMO flow with a centrifugal pump may be caused by a preload decrease or afterload augmentation. Decreases in preload may be caused by several factors, such as bleeding or hypovolemia. Afterload is usually influenced by systemic vascular resistance (SVR) or kinks in the atrial cannula, or a thrombus in the oxygenator.
For peripheral VA-ECMO, placement of a distal perfusion cannula (DPC) is recommended to prevent limb ischemia (39). Flow through a DPC can be monitored by an ultrasound flow meter. Generally, flow through a DPC changes in a linear positive correlation with ECMO blood flow, and variables that influence ECMO flow could also influence DPC flow. There is no agreement for a minimal recommended DPC flow to maintain limb perfusion. In our center, a 6–8 Fr distal perfusion cannula (DPC) is routinely used for adults, with a minimal 150 mL/min flow recommended to prevent limb ischemia.
Monitoring native CO
Patients with VA-ECMO support are characterized by end-organ hypoperfusion caused by low CO and hypotension. Advanced hemodynamic monitoring is important in detecting native CO and guiding treatment. Several hemodynamic monitoring methods for the assessment of native CO have not yet been validated, and should be cautiously used in patients with VA-ECMO support (Table 1).
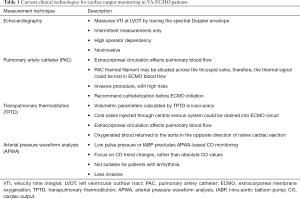
Full table
Echocardiography is a widely used noninvasive method of hemodynamic assessment (40). Using this approach, CO can be estimated by several methods. The most frequently used method involves measuring blood flow velocity (Doppler technique) at the left ventricular outflow tract (LVOT), thus, providing a beat-to-beat measurement of SV (41).
The pulmonary artery catheter (PAC) also measures CO. However, the thermodilution method does not allow accurate CO measurements in patients with VA-ECMO, as firstly, extracorporeal circulation affects pulmonary blood flow, and secondly thermal signals could be lost in ECMO blood flow, since part of the PACs thermal filament may be situated across the tricuspid valve (42). Although limited for CO measurement, the PAC still provides valuable hemodynamic information such as pulmonary artery wedge pressure (PAWP), which are regarded as indicators of LV distention.
Transpulmonary thermodilution (TPTD) provides a comprehensive hemodynamic assessment for critically-ill patients (43). Redwan and colleagues reported that low-flow veno-venous ECMO (VV-ECMO) did not influence hemodynamic monitoring, when using TPTD (44). However, TPTD is inappropriate for CO measurements in patients with VA-ECMO, since unknown levels of cold saline bolus are drained into the ECMO circuit, generating inaccurate calculations.
Arterial pressure waveform analysis (APWA) devices, such as Vigileo/FloTrac systems (Edwards Lifesciences, Irvine, CA, USA) provide real-time CO measurements by deriving stroke volumes from the arterial pressure curve. These devices are validated during perioperative settings despite working without external calibration (45,46). However, APWA systems become unreliable when major hemodynamic or vasomotor tone changes exist (47,48). VA-ECMO patients often experience large changes in arterial resistance, either spontaneously or due to vasopressors, and can present little-to-no pulsatility (49,50). Hence, these devices are unsuitable in these situations. In some clinical settings, i.e., during an ECMO weaning process, which is characterized by low ECMO blood flow, low vasopressor doses and relative normal pulse pressure (PP), APWA devices may play a role, but their validation is essential.
Monitoring MAP
A sufficient MAP is essential in maintaining end-organ perfusion. MAP is a product of total CO and SVR. In VA-ECMO patients, MAP increases may be achieved by increasing either CO or SVR, using vasoactive drugs. As total CO is composed of VA-ECMO flow and native CO, increasing ECMO flow or native CO could increase MAP. Although there is insufficient evidence to recommend optimal MAP objectives, an initial MAP of >60 mmHg may be reasonable, and should be adjusted according to individual circumstances. As MAP increases are related to increases in afterload, balances between the effects of increased afterload and adequate tissue perfusion should be weighed.
Monitoring microcirculation
VA-ECMO enables rapid improvements in systemic hemodynamic parameters, such as blood pressure, total CO and SvO2. However, there is no guarantee that systemic hemodynamic normalization also improves microcirculatory and tissue perfusion. The incoherence between macro- and micro-circulation is a common pathophysiology phenomenon in cardiogenic shock patients (51-53). At present, hand-held video microscopy is a promising tool, which assesses microcirculation disturbances at bedside. Only certain anatomical regions, such as sublingual areas can be monitored due to technical restrictions. Previous studies have shown that sublingual microcirculatory disturbances are predominant in sepsis or cardiogenic shock patients, and are associated with mortality (54-56). The use of sublingual microcirculatory monitoring in patients with VA-ECMO support is now emerging. Perfused vessel density (PVD) of sublingual areas at VA-ECMO initiation is associated with mortality in patients with cardiogenic shock (57). The inability to restore microcirculation parameters, such as perfused small-vessel densities, small-vessel densities, and percent perfused vessels during the first 24 hours, was associated with mortality in VA-ECMO patients with refractory cardiogenic shock (58). Sustained values for total vessel density (TVD) and PVD during a 50% ECMO flow reduction, were more specific and sensitive for predicting successful weaning from VA-ECMO, than echocardiographic parameters (59). However, the widespread use of sublingual microcirculatory monitoring is restricted since measurements are time-consuming and expensive, and they require an experienced operator, as well post-monitoring complex analysis (60). Further studies are warranted to determine the role of sublingual microcirculatory monitoring in the management of patients during VA-ECMO support.
Cardiac function assessments
Rhythm
Cardiac arrhythmias often compromise native cardiac function, and cause hemodynamic instability, which may exacerbate a failing heart. Cardiac arrhythmia can arise due to myocardial ischemia, pharmacological effects, electrolyte disturbances and occult bleeding. Certain arrhythmias such as ventricular fibrillation is life-threatening, and require urgent management, including direct current cardioversion, antiarrhythmic medication, or pacing.
Pulsatility
Patients supported with VA-ECMO should be monitored with an arterial line, ideally placed in the right radial artery. Arterial line placement allows PP monitoring (pulsatility on atrial waveform) as an indication of LV contractility. Absent or low pulsatility indicate decreases in LV stroke volume, leading to blood stasis and an increased risk of thrombus formation. In contrast, higher pulsatility indicates possible myocardial recovery during VA-ECMO support. Moreover, arterial blood gas analysis from the right radial artery could be indicative of oxygen supply of cerebral blood flow in the setting of peripheral cannulation.
Cardiac biomarkers
Traditional cardiac biomarkers such as cardiac troponin I (cTnI), cardiac troponin T (cTnT) and serum N-Terminal pro-brain natriuretic peptide (NT-proBNP) are prognostic outcome predictors for cardiogenic shock (61-63). These cardiac biomarkers can be used to assess ventricular function, and serial measurements may be helpful in monitoring ventricular recovery.
Echocardiography
Echocardiography is recommended as the first-line evaluation tool in patients with VA-ECMO (64). Not only is cardiac chamber size and global function assessed, but valve functions can also be evaluated, of which aortic valve opening is a critically important aspect. The persistently closed aortic valve, and increased LV dimensions may signify LV distention, thereby indicating poor LV recovery (65). Similarly, routine evaluations of biventricular functions enable earlier recovery (64). As ECMO flow decreases, an absence of biventricular dilatation indicates cardiac recovery.
PAC
The PAC provide CO measurements, filling pressures of left and right ventricles (PAWP and right atrial pressure), and right ventricular afterload (pulmonary artery pressure), and SvO2 (66). Although the PAC has limitations for CO measurements during VA-ECMO, it still provides valuable hemodynamic information such as PAWP, which is one of the indicators for LV distention. Central venous pressure (CVP), which correlates with right atrial pressure, is typically used to assess volume status and cardiac preload. Normally, CVP is low due to continuous venous drainage during VA-ECMO support. Higher CVP in the VA-ECMO setting could hint at venous congestion or ventricle dysfunction. It should be borne in mind that CVP can be impacted by several elements such as cardiac function, mechanical ventilation, position of the central catheter tip and vasoactive agents (67,68).
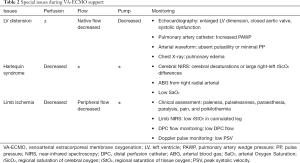
Special issues
LV distention
If LV contractile function is severely impaired, and LV afterload increases, the aortic valve tends to be closed. In this situation, blood accumulates in the LV chamber, ultimately leading to fatal thromboembolic complications (69). A persistently closed aortic valve indicates LV overload, and extremely high levels of LV end-diastolic pressure (LVEDP) and left atrial pressure (LAP). An increased end-diastolic pressure (EDP) results in elevated vessel wall stress and myocardial oxygen demand, creating a detrimental feedback loop for LV function. In addition, increased LAP and PAWP are detrimental to native blood oxygen saturation from the lungs, and result in progressive pulmonary edema. LV distention is regarded as the main cause of poor LV recovery, and failure to wean off VA-ECMO (65).
The identification of LV distention during VA-ECMO support is critical for patient management (Table 2). Several approaches have been used to monitor and recognize high risk patients. Firstly, echocardiography is the most common approach for assessing ventricle function. Echocardiography assesses not only the extent and duration of aortic valve opening, but also changes in LV dimensions. As the LV end-diastolic pressure-volume relationship is nonlinear, large increases in LV EDP may cause only minor increases in LV EDV. The use of LV chamber size as an index of LV distention or LV EDP may be insensitive. Secondly, the most direct and time-sensitive approach in detecting LV loading and the degree of pulmonary congestion, is using a PAC, which measures either pulmonary artery pressure (PAP) or PAWP. Thirdly, the presence and degree of aortic valve opening may be recognized on the arterial pulse pressure tracing. With increasing ECMO flow, MAP increases but SV and PP decrease, indicating that the duration of aortic valve opening is becoming shorter. A lack of pulsatility on arterial waveforms may signify a closed aortic valve and worsening myocardial function, while the appearance of pulsatility or increased PP may signify aortic valve opening, and cardiac functional recovery. Fourthly, diffuse infiltration on a chest X-ray indicates pulmonary edema, and extremely high PAWP levels. However, these radiographic findings are nonspecific, and could be due to other pathologies, such as infection or acute respiratory distress syndrome. Once there is evidence for LV distension and progressive pulmonary edema, LV decompression should be considered. However, optimal indications and timings are currently unknown.
Full table
Harlequin syndrome
In VA-ECMO patients, native left ventricular blood forward flow mixes with ECMO circuit retrograde blood flow. In an impaired pulmonary function setting, the brain, heart, and upper extremities receive poorly oxygenated blood, and may appear cyanotic; while the lower extremities, which receive fully oxygenated blood from the ECMO circuit, appear pink. This phenomenon has been termed Harlequin syndrome (Table 2). Areas supplied by the brachiocephalic artery, such as the right side of the face or the right upper extremity, should be monitored. Arterial blood gas analysis, pulse oximetry, or tissue oximetry are usually performed to monitor oxygenation. As arterial blood gas sampling at various locations provide different PaO2 values, a right radial arterial line should be recommended as the optimal choice in detecting coronary and cerebral hypoxemia. Once Harlequin syndrome is determined, measures, including ventilator setting adjustments, transforming to V-AV ECMO, or central cannulation should be considered for adequate oxygenation (70).
Limb ischemia
Lower extremity ischemia is a critical complication occurring in 10–70% of patients during peripheral VA-ECMO support (71,72). Severe limb ischemia can lead to compartment syndrome, which may require fasciotomy or even limb amputation. Reduced blood flow and inadequate oxygen delivery to the lower extremities induce ischemia via multiple factors, including femoral vessel damage, high vasopressor doses, a larger cannula diameter and underlying arterial disease.
Monitoring the cannulated leg to ensure adequate perfusion is essential (Table 2). Typical symptoms of limb ischemia include paleness, pulselessness, paraesthesia, paralysis, pain, and poikilothermia (73). Doppler ultrasonography can monitor peak systolic velocity (PSV) of distal arteries in VA-ECMO patients (74), however a lack of pulsatility renders PSV unreliable, especially in fully supported ECMO patients. Tissue oximetry is a promising tool that provides quantitative measures of limb oxygenation, independent of the pulsatile blood flow. Lower rStO2 levels in the cannulated leg or large differences in rStO2 levels between the legs at the time of ECMO insertion, may indicate lower extremity ischemia (36,37). Elevated creatinine, phosphokinase or lactate levels can also be used to diagnose limb ischemia. The prophylactic use of distal perfusion catheters can also effectively reduce the incidence of limb ischemia (75,76). If limb ischemia persists, the optimization of ECMO flow or vasopressor administration, and further surgical intervention may be considered.
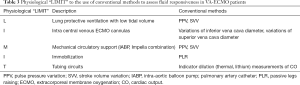
Prediction of fluid responsiveness in patients with VA-ECMO
Fluid therapy is an important aspect of VA-ECMO management. Since positive fluid balances are associated with poor outcomes (77-79), predicting fluid responsiveness is important in avoiding unnecessary fluid administration.
CVP and other static markers of cardiac preload such as PAWP have proved unreliable in predicting fluid responsiveness in critically ill patients. Dynamic markers exploring intra-tidal cyclic changes in hemodynamics, such as pulse pressure variations (PPV) and stroke volume variations (SVV) during mechanical ventilation, accurately predict fluid responsiveness (80-83). It should be noted they are unreliable under certain conditions, such as spontaneous breathing (even in an intubated patient), cardiac arrhythmias, high heart rate to respiratory rate ratios, intra-abdominal hypertension, and low tidal volume/lung compliance (84-87). Variations in vena cava or internal jugular vein diameters also accurately reflect fluid responsiveness, and share many of the same limitations as PPV/SVV (88-91).
Unfortunately, there is no evidence on fluid responsiveness assessments in patients with VA-ECMO. There is a physiological “LIMIT” to the use of conventional methods to assess fluid responsiveness in VA-ECMO patients (Table 3). Firstly, patients with VA-ECMO often have a lung protective ventilation strategy, which lowers the amplitude of changes in intrathoracic pressure. Secondly, drainage cannulation placed in inferior vena cava (IVC) impedes the application of vena cava diameter variation. Thirdly, retrograde blood supplied by the ECMO circuit and impaired LV contractility, often lead to an absent or low pulsatility on arterial waveforms. Sometimes other mechanical circulatory supports (such as intra-aortic balloon pump) may be combined with VA-ECMO. In these instances, PPV is inapplicable and APWA techniques are limited in their use.
Full table
The passive leg raising (PLR) test is frequently used as a reliable provocative test to detect preload responsiveness. This test, as a “reversible volume challenge”, provides an amount of around 300 mL blood and can be repeated as frequently as required without infusing any fluids (92). Recent studies have confirmed PLR as a reliable method in predicting fluid responsiveness, with few limitations (93,94). However, leg raising is often impractical, with the immobilization of lower extremities during peripheral VA-ECMO support. The Trendelenburg maneuver may be a promising alternative to transiently increasing preload and detecting fluid responsiveness. Yonis et al. demonstrated that the Trendelenburg maneuver was reliable in predicting fluid responsiveness in acute respiratory distress syndrome (ARDS) patients in a prone position (95). A study predicting fluid responsiveness using the Trendelenburg maneuver in VA-ECMO patients is ongoing (NCT03553459).
VA-ECMO weaning and hemodynamic monitoring
Weaning from VA-ECMO is proposed when the patient manifests signs of partial or full circulatory recovery. Currently, there are no standard VA-ECMO weaning strategies. Of the reported various weaning strategies, some are institution dependent and are limited by small sample size and are retrospective in nature (96-98). PP appears to be an important clinical parameter associated with successful weaning (99). Echocardiographic assessments provide robust information for successful weaning in this setting. Improved ventricular contractility and consistent opening of the aortic valve via echocardiography, provides promising indications of cardiac recovery. With ECMO flow gradually decreased, LV and RV functions and hemodynamic parameters should be continuously monitored. If no signs of significant hypotension or LV or RV distension are observed, a further decrease in ECMO flow can be attempted. If a patient has a left ventricular ejection fraction (LVEF) of ≥20–25%, an aortic velocity-time integral (VTI) of ≥10 cm, and a spectral tissue Doppler lateral mitral annulus peak systolic (TDSa) ≥6 cm/s under minimal ECMO support, the removal of ECMO support should be considered (98). In addition, sublingual microcirculatory monitoring may also provide valuable information during the VA-ECMO weaning process (59). Intensivists should note a successful weaning is difficult to predict with certainty until ECMO support is completely removed, since cardiac dysfunction may be masked even under minimal ECMO flow.
SummaryOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
Ten items that ICU specialists must know about hemodynamic monitoring in VA-ECMO patients were summarized in Table 4. Based on physiological changes during VA-ECMO, hemodynamic monitoring plays a crucial role in individualized therapy. Despite no empirical evidence for hemodynamic monitoring during VA-ECMO, we suggest three elements of hemodynamic monitoring: perfusion, flow and heart. Though variables of hemodynamic monitoring tools are used across clinical practice, intensivists should be aware of the advantages and limitations of these techniques.

Full table
AcknowledgmentsOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
A novel coronavirus (COVID-19) outbreak is emerging in Wuhan, China since December 2019. Five authors (YS, KL, JLZ, ZL and GWT) of the review are fighting against the epidemic situation in Wuhan Now. Wish them a safe return.
Funding: This article was supported by grants from the Research Funds of Zhongshan Hospital (2019ZSQN13, 2019ZSYXQN34, 2019ZSXMYS04 and XYYX201922) and the Research Fund of Shanghai Municipal Health Commission (2019ZB0105).
FootnoteOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-wei Tu) for the series “Hemodynamic Monitoring in Critically Ill Patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.186). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. GWT served as the unpaid Guest Editor of the series and serves as an unpaid Section Editor of Annals of Translational Medicine from May 2018 to Sep 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- VA-ECMO pathophysiology
- Hemodynamic monitoring in VA-ECMO patients
- Summary
- Acknowledgments
- Footnote
- References
- Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;73:698-716. [Crossref] [PubMed]
- Truby L, Mundy L, Kalesan B, et al. Contemporary Outcomes of Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock at a Large Tertiary Care Center. Asaio J 2015;61:403-9. [Crossref] [PubMed]
- Suguta M, Hoshizaki H, Anno M, et al. Right ventricular infarction with cardiogenic shock treated with percutaneous cardiopulmonary support: a case report. Jpn Circ J 1999;63:813-5. [Crossref] [PubMed]
- Burkhoff D, Sayer G, Doshi D, et al. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663-74. [Crossref] [PubMed]
- Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011;37:801-7. [Crossref] [PubMed]
- Coudroy R, Jamet A, Frat JP, et al. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med 2015;41:452-9. [Crossref] [PubMed]
- Brunauer A, Kokofer A, Bataar O, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study. J Crit Care 2016;35:105-9. [Crossref] [PubMed]
- Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med 2014;40:958-64. [Crossref] [PubMed]
- Zampieri FG, Damiani LP, Bakker J, et al. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels Among Patients with Septic Shock: A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med 2020;201:423-9. [Crossref] [PubMed]
- Hernández G, Ospina-Tascon GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019;321:654-64. [Crossref] [PubMed]
- Boerma EC, Kuiper MA, Kingma WP, et al. Disparity between skin perfusion and sublingual microcirculatory alterations in severe sepsis and septic shock: a prospective observational study. Intensive Care Med 2008;34:1294-8. [Crossref] [PubMed]
- Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014;18:503. [Crossref] [PubMed]
- Yang L, Fan Y, Lin R, et al. Blood Lactate as a Reliable Marker for Mortality of Pediatric Refractory Cardiogenic Shock Requiring Extracorporeal Membrane Oxygenation. Pediatr Cardiol 2019;40:602-9. [Crossref] [PubMed]
- Mungan İ, Kazanci D, Bektas S, et al. Does lactate clearance prognosticates outcomes in ECMO therapy: a retrospective observational study. Bmc Anesthesiol 2018;18:152. [Crossref] [PubMed]
- Bonizzoli M, Lazzeri C, Cianchi G, et al. Serial Lactate Measurements as a Prognostic Tool in Venovenous Extracorporeal Membrane Oxygenation Support. Ann Thorac Surg 2017;103:812-8. [Crossref] [PubMed]
- Li CL, Wang H, Jia M, et al. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: A retrospective observational study. J Thorac Cardiovasc Surg 2015;149:1445-50. [Crossref] [PubMed]
- Slottosch I, Liakopoulos O, Kuhn E, et al. Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care 2017;42:35-41. [Crossref] [PubMed]
- De Backer D. Detailing the cardiovascular profile in shock patients. Crit Care 2017;21:311. [Crossref] [PubMed]
- Pölönen P, Ruokonen E, Hippelainen M, et al. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 2000;90:1052-9. [Crossref] [PubMed]
- Boulain T, Garot D, Vignon P, et al. Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care 2014;18:609. [Crossref] [PubMed]
- Gallet R, Lellouche N, Mitchell-Heggs L, et al. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch Cardiovasc Dis 2012;105:5-12. [Crossref] [PubMed]
- Ladakis C, Myrianthefs P, Karabinis A, et al. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration 2001;68:279-85. [Crossref] [PubMed]
- Tahvanainen J, Meretoja O, Nikki P. Can central venous blood replace mixed venous blood samples? Crit Care Med 1982;10:758-61. [Crossref] [PubMed]
- Vincent JL, De Backer D. From Early Goal-Directed Therapy to Late(r) Scvo2 Checks. Chest 2018;154:1267-9. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Reinhart K, Rudolph T, Bredle DL, et al. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest 1989;95:1216-21. [Crossref] [PubMed]
- Protti A, Masson S, Latini R, et al. Persistence of Central Venous Oxygen Desaturation During Early Sepsis Is Associated With Higher Mortality: A Retrospective Analysis of the ALBIOS Trial. Chest 2018;154:1291-300. [Crossref] [PubMed]
- Han L, Zhang Y, Zhang Y, et al. Risk factors for refractory septic shock treated with VA ECMO. Ann Transl Med 2019;7:476. [Crossref] [PubMed]
- Wagner K, Risnes I, Abdelnoor M, et al. Is it possible to predict outcome in cardiac ECMO? Analysis of preoperative risk factors. Perfusion 2007;22:225-9. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, Parise O, et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Crit Care Med 2017;45:1389-97. [Crossref] [PubMed]
- Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med 2016;44:e964-72. [Crossref] [PubMed]
- Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med 2013;2:40-7. [Crossref] [PubMed]
- Risnes I, Wagner K, Nome T, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg 2006;81:1401-6. [Crossref] [PubMed]
- Khan I, Rehan M, Parikh G, et al. Regional Cerebral Oximetry as an Indicator of Acute Brain Injury in Adults Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation-A Prospective Pilot Study. Front Neurol 2018;9:993. [Crossref] [PubMed]
- Pozzebon S, Blandino OA, Franchi F, et al. Cerebral Near-Infrared Spectroscopy in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. Neurocrit Care 2018;29:94-104. [Crossref] [PubMed]
- Kim DJ, Cho YJ, Park SH, et al. Near-Infrared Spectroscopy Monitoring for Early Detection of Limb Ischemia in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation. Asaio J 2017;63:613-7. [Crossref] [PubMed]
- Steffen RJ, Sale S, Anandamurthy B, et al. Using near-infrared spectroscopy to monitor lower extremities in patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg 2014;98:1853-4. [Crossref] [PubMed]
- Keebler ME, Haddad EV, Choi CW, et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail 2018;6:503-16. [Crossref] [PubMed]
- Makdisi G, Makdisi T, Wang IW. Use of distal perfusion in peripheral extracorporeal membrane oxygenation. Ann Transl Med 2017;5:103. [Crossref] [PubMed]
- Papolos A, Narula J, Bavishi C, et al. U.S. Hospital Use of Echocardiography: Insights From the Nationwide Inpatient Sample. J Am Coll Cardiol 2016;67:502-11. [Crossref] [PubMed]
- Jozwiak M, Monnet X, Teboul JL. Monitoring: from cardiac output monitoring to echocardiography. Curr Opin Crit Care 2015;21:395-401. [Crossref] [PubMed]
- Reis Miranda D, van Thiel R, Brodie D, et al. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2015;191:346-8. [Crossref] [PubMed]
- Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care 2017;21:147. [Crossref] [PubMed]
- Redwan B, Ziegeler S, Freermann S, et al. Single-Site Low-Flow Veno-Venous Extracorporeal Lung Support Does Not Influence Hemodynamic Monitoring by Transpulmonary Thermodilution. Asaio J 2016;62:454-7. [Crossref] [PubMed]
- Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016;42:1350-9. [Crossref] [PubMed]
- Slagt C, Malagon I, Groeneveld AB. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth 2014;112:626-37. [Crossref] [PubMed]
- Meng L, Tran NP, Alexander BS, et al. The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth Analg 2011;113:751-7. [Crossref] [PubMed]
- Hamzaoui O, Monnet X, Richard C, et al. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 2008;36:434-40. [Crossref] [PubMed]
- Zotzmann V, Rilinger J, Lang CN, et al. Epinephrine, inodilator, or no inotrope in venoarterial extracorporeal membrane oxygenation implantation: a single-center experience. Crit Care 2019;23:320. [Crossref] [PubMed]
- Meani P, Delnoij T, Raffa GM, et al. Protracted aortic valve closure during peripheral veno-arterial extracorporeal life support: is intra-aortic balloon pump an effective solution? Perfusion 2019;34:35-41. [Crossref] [PubMed]
- Jung C, Ferrari M, Rodiger C, et al. Evaluation of the sublingual microcirculation in cardiogenic shock. Clin Hemorheol Microcirc 2009;42:141-8. [Crossref] [PubMed]
- De Backer D, Creteur J, Dubois MJ, et al. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 2004;147:91-9. [Crossref] [PubMed]
- Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015;19 Suppl 3:S8. [Crossref] [PubMed]
- Massey MJ, Hou PC, Filbin M, et al. Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care 2018;22:308. [Crossref] [PubMed]
- den Uil CA, Lagrand WK, van der Ent M, et al. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2010;31:3032-9. [Crossref] [PubMed]
- den Uil CA, Maat AP, Lagrand WK, et al. Mechanical circulatory support devices improve tissue perfusion in patients with end-stage heart failure or cardiogenic shock. J Heart Lung Transplant 2009;28:906-11. [Crossref] [PubMed]
- Kara A, Akin S, Dos RMD, et al. Microcirculatory assessment of patients under VA-ECMO. Crit Care 2016;20:344. [Crossref] [PubMed]
- Chommeloux J, Montero S, Franchineau G, et al. Microcirculation Evolution in Patients on Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock. Crit Care Med 2020;48:e9-17. [Crossref] [PubMed]
- Akin S, Dos RMD, Caliskan K, et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care 2017;21:265. [Crossref] [PubMed]
- Huber W, Zanner R, Schneider G, et al. Assessment of Regional Perfusion and Organ Function: Less and Non-invasive Techniques. Front Med (Lausanne) 2019;6:50. [Crossref] [PubMed]
- Duchnowski P, Hryniewiecki T, Kusmierczyk M, et al. High-Sensitivity Troponin T Predicts Postoperative Cardiogenic Shock Requiring Mechanical Circulatory Support in Patients with Valve Disease. Shock 2020;53:175-8. [Crossref] [PubMed]
- Januzzi JL, Morss A, Tung R, et al. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: a prospective cohort study. Crit Care 2006;10:R37. [Crossref] [PubMed]
- Chong SZ, Fang CY, Fang HY, et al. Associations with the In-Hospital Survival Following Extracorporeal Membrane Oxygenation in Adult Acute Fulminant Myocarditis. J Clin Med 2018. [Crossref] [PubMed]
- Douflé G, Roscoe A, Billia F, et al. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015;19:326. [Crossref] [PubMed]
- Kurihara H, Kitamura M, Shibuya M, et al. Effect of transaortic catheter venting on left ventricular function during venoarterial bypass. Asaio J 1997;43:M838-41. [Crossref] [PubMed]
- Pour-Ghaz I, Manolukas T, Foray N, et al. Accuracy of non-invasive and minimally invasive hemodynamic monitoring: where do we stand? Ann Transl Med 2019;7:421. [Crossref] [PubMed]
- Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part I: basic concepts. Intensive Care Med 2009;35:45-54. [Crossref] [PubMed]
- Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology 2008;108:735-48. [Crossref] [PubMed]
- Weber C, Deppe AC, Sabashnikov A, et al. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion 2018;33:283-8. [Crossref] [PubMed]
- Sorokin V, MacLaren G, Vidanapathirana PC, et al. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: the potential dynamic process of organ support and importance of hybrid modes. Eur J Heart Fail 2017;19 Suppl 2:75-83. [Crossref] [PubMed]
- Yang F, Hou D, Wang J, et al. Vascular complications in adult postcardiotomy cardiogenic shock patients receiving venoarterial extracorporeal membrane oxygenation. Ann Intensive Care 2018;8:72. [Crossref] [PubMed]
- Pozzi M, Koffel C, Djaref C, et al. High rate of arterial complications in patients supported with extracorporeal life support for drug intoxication-induced refractory cardiogenic shock or cardiac arrest. J Thorac Dis 2017;9:1988-96. [Crossref] [PubMed]
- Pratt GH, Krahl E. Surgical therapy for the occluded artery. Am J Surg 1954;87:722-9. [Crossref] [PubMed]
- Breeding J, Hamp T, Grealy R, et al. Effects of extracorporeal membrane oxygenation pump flow, backflow cannulae, mean arterial blood pressure, and pulse pressure on Doppler-derived flow velocities of the lower limbs in patients on peripheral veno-arterial extracorporeal membrane oxygenation: A pilot study. Aust Crit Care 2019;32:206-12. [Crossref] [PubMed]
- Lamb KM, DiMuzio PJ, Johnson A, et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J Vasc Surg 2017;65:1074-9. [Crossref] [PubMed]
- Juo YY, Skancke M, Sanaiha Y, et al. Efficacy of Distal Perfusion Cannulae in Preventing Limb Ischemia During Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Artif Organs 2017;41:E263-73. [Crossref] [PubMed]
- Selewski DT, Askenazi DJ, Bridges BC, et al. The Impact of Fluid Overload on Outcomes in Children Treated With Extracorporeal Membrane Oxygenation: A Multicenter Retrospective Cohort Study. Pediatr Crit Care Med 2017;18:1126-35. [Crossref] [PubMed]
- Alobaidi R, Lequier L. Fluid Overload and Extracorporeal Membrane Oxygenation: Is Renal Replacement Therapy a Buoy or an Anchor? Pediatr Crit Care Med 2017;18:1181-2. [Crossref] [PubMed]
- Staudacher DL, Gold W, Biever PM, et al. Early fluid resuscitation and volume therapy in venoarterial extracorporeal membrane oxygenation. J Crit Care 2017;37:130-5. [Crossref] [PubMed]
- Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care 2014;18:650. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000;162:134-8. [Crossref] [PubMed]
- Ma GG, Tu GW, Zheng JL, et al. Changes in Stroke Volume Variation Induced by Passive Leg Raising to Predict Fluid Responsiveness in Cardiac Surgical Patients With Protective Ventilation. J Cardiothorac Vasc Anesth 2020;34:1526-33. [Crossref] [PubMed]
- Myatra SN, Prabu NR, Divatia JV, et al. The Changes in Pulse Pressure Variation or Stroke Volume Variation After a "Tidal Volume Challenge" Reliably Predict Fluid Responsiveness During Low Tidal Volume Ventilation. Crit Care Med 2017;45:415-21. [Crossref] [PubMed]
- Liu Y, Wei LQ, Li GQ, et al. Pulse Pressure Variation Adjusted by Respiratory Changes in Pleural Pressure, Rather Than by Tidal Volume, Reliably Predicts Fluid Responsiveness in Patients With Acute Respiratory Distress Syndrome. Crit Care Med 2016;44:342-51. [Crossref] [PubMed]
- Díaz F, Erranz B, Donoso A, et al. Influence of tidal volume on pulse pressure variation and stroke volume variation during experimental intra-abdominal hypertension. Bmc Anesthesiol 2015;15:127. [Crossref] [PubMed]
- Monnet X, Bleibtreu A, Ferre A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med 2012;40:152-7. [Crossref] [PubMed]
- Vignon P, Repesse X, Begot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med 2011;26:116-24. [Crossref] [PubMed]
- Feissel M, Michard F, Faller JP, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 2004;30:1834-7. [Crossref] [PubMed]
- Ma GG, Hao GW, Yang XM, et al. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann Intensive Care 2018;8:6. [Crossref] [PubMed]
- Jabot J, Teboul JL, Richard C, et al. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 2009;35:85-90. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935-47. [Crossref] [PubMed]
- Cherpanath TG, Hirsch A, Geerts BF, et al. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta-Analysis of 23 Clinical Trials. Crit Care Med 2016;44:981-91. [Crossref] [PubMed]
- Yonis H, Bitker L, Aublanc M, et al. Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care 2017;21:295. [Crossref] [PubMed]
- Aissaoui N, El-Banayosy A, Combes A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med 2015;41:902-5. [Crossref] [PubMed]
- Cavarocchi NC, Pitcher HT, Yang Q, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg 2013;146:1474-9. [Crossref] [PubMed]
- Aissaoui N, Luyt CE, Leprince P, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 2011;37:1738-45. [Crossref] [PubMed]
- Park BW, Seo DC, Moon IK, et al. Pulse pressure as a prognostic marker in patients receiving extracorporeal life support. Resuscitation 2013;84:1404-8. [Crossref] [PubMed]


