
Preemptive kidney support: an optimal practice or a good theory?
A recent discussion on Critical Care Nephrology nomenclature, promoted by the Kidney Disease: Improving Global Outcome (KDIGO) workgroup, endorsed a modification from the term “renal replacement” to “kidney support” (https://kdigo.org/conferences/aki-conference/). Driving this recommendation is the fact that when clinicians deliver dialysis, they are not literally substituting the function of the kidney with an artificial technique, since this would be impossible. On the other side, the prescription of a kidney support therapy (KST) might interrupt the progression of kidney injury, before any dysfunction has become irreversible (e.g., against venous congestion), but also limiting detrimental effects in an attempt to restore renal function (1) (e.g., by contributing to reduce vasopressor doses and cardiac afterload, ultimately optimizing renal microcirculation). The term support, however, also encompasses the idea of limiting secondary non-renal organ injury (organ crosstalk). The KST paradigm shift focuses on acute dialysis as a specific treatment aimed not only at the replication of certain renal functions (namely, small solute clearance and fluid output) but also to a more comprehensive therapy, applied to patients with multiple organ dysfunction. Treating acute kidney injury (AKI) in critically ill patients entails supporting and benefitting the whole organism (2).
In this context, several favorable consequences could be considered when a proactive KST approach is applied: first, optimization of fluid balance and organ decongestion (3), before fluid overload contributes to organ function deterioration (4); second, delivery of an intravenous parenteral nutrition (5), regardless of the prescribed volume, in order to meet a specific caloric and aminoacidic intake; third, immunomodulation and attenuation of inflammatory cascade (6) by clearing inflammatory mediators in both septic and surgical patients with signs of systemic over-inflammation and risks of organ injury; fourth, re-establishment of acid-base and electrolytes balance by means of balanced buffered dialysis and replacement fluids to improve catecholamines affinity to their receptors and to reverse vasoplegia and hypotension (7).
This concept has led to the idea, described in the Annals of Translational Medicine by Guo-Wei Tu and coauthors, of “pre-emptive KST” (8): a timely support was applied to a specific cohort of post cardiac surgery patients based on a previous authors’ experience that early KST was “associated with lower hospital mortality, and faster and more frequent recovery of renal function” (9). Specifically, the authors, with a before-after (“historically controlled”) study, compared two different periods at their institution, one before (Period A), and one after (Period B), a “pre-emptive” renal replacement therapy (RRT) protocol was initiated, in order to verify if any outcome differences were evident in their patients. Authors’ definition of early KST was (I) AKI in the absence of “conventional” (urgent or emergent) indications for RRT; (II) persistent hypotension [mean arterial pressure (MAP) <65 mmHg for more than 6 h] with high-dose vasoactive drugs despite preload optimization; (III) low probability of rapid renal recovery according to the judgment of the intensivists and nephrologists. In essence, their main target was to treat patients with mild AKI and post-cardiotomy cardiogenic shock. Of particular interest, regarding the case mix presented by the authors, is the fact that they accurately selected the patients who received pre-emptive KST: of 12,000 screened cardiac surgery patients, only about 150 appeared to have the entry criteria and the half were included in the pre-emptive period. Also of interest was that the compared populations seemed to have similar clinical characteristics at baseline, whereas they showed significant differences at the beginning of the dialytic treatment: pre-emptive patients had accumulated less fluid, were receiving a lower dose of vasopressors, had a slightly higher mean MAP, apparently closer to the entry threshold of 65 mmHg, and were less severely ill compared to patients of Period A. These aspects might suggest that populations with different clinical pictures were compared (the authors did not attempt any multivariate adjustment of their data). On the other side, earlier initiation of RRT in the course of AKI may have prevented further worsening of electrolyte, acid-base, and fluid-balance disturbances: two different phases of a similar critical illness were treated, at different stages of the progression of hemodynamic instability and, maybe, of organ damage (10).
In fact, one of the most challenging clinical questions of the treatment of critically ill patients, especially as far as extracorporeal purification is concerned, is to appraise how the disease is going to evolve. In the case of AKI, several authors have attempted to understand if and when a patient might need KST or if they could avoid this unnecessary invasive treatment (11). This issue is the most addressed topic on the interpretation of the three randomized controlled trials recently conducted on timing of RRT (11). Recently, an interesting meta-analysis showed that, although some renal biomarkers, namely neutrophil gelatinase associated lipocalin (NGAL) and tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7), have shown some promise in anticipating the requirement of acute dialysis, their application in a clinical routine with this indication is not feasible yet (12). It is possible that research in this field will contribute to the development of novel biomarkers with the specific purpose of guiding clinicians towards the difficult choice of starting an early KST or a standard RRT. Also, of interest is the attempt of other authors to predict, through the furosemide stress test (i.e., the patients’ diuresis after a furosemide bolus during the following 120 minutes), both the evolution of an initial AKI episode (13) and the probability of a future need of KST (14). As a matter of fact, in the study by Guo-Wei Tu and collaborators, the time between surgery and dialysis was 20 hours shorter in the early group and mortality and renal function recovery significantly improved. Unfortunately, the authors did not attempt a direct comparison between the two groups but only observed, in each population, how the vital parameter modification behaved: hemodynamics showed improvement in MAP and reduction of central venous pressure only in the pre-emptively treated patients, with an associated reduction on vasopressors doses. Again, due to the inherent limitations of a retrospective study, the authors were not able to test the “biological effects” of their KST approach and its clinical plausibility. We do not have any information on echocardiography, advanced hemodynamic investigations (e.g., cardiac output or wedge pressure), fluid balance, acid base, and lung function modifications during the two treatments. However, renal function appeared to recover in a quicker and more efficient way with the pre-emptive approach: kidneys certainly benefit from timely KST, being capsulated organs that mostly suffer from organ congestion and fluid overload (15,16).
As a matter of fact, trials on proactive or anticipated acute dialysis have found controversial clinical results in the critical care nephrology literature during last 20 years (17-20). Even if the phenotypes of multiorgan failure requiring intensive care support may appear similar among different patients, significant differences from case to case are indeed present. This is essentially due to the fact that critical illness requiring organ support (i.e., mechanical ventilation, vasoactive drugs, dialysis, etc.) is constituted by an extremely broad series of admission diseases (i.e., medical and surgical, infective or auto-immune, elective or urgent, etc.), pathophysiology, complications and different severity and involvement of other organs. Hence, it has been impossible, so far, to find an unequivocal indication, specific technology, ideal prescription and exact timing for pre-emptive KST.
It must be remarked, however, that among current trials analyzing the timing of CRRT, the Effect of Early vs. Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury (ELAIN) Randomized Clinical Trial (19), that included many post-cardiac surgery patients and that apparently had a similar design to the one of Guo-Wei Tu and coauthors, showed a significant benefit of early KST initiation. It is possible that cardiac surgery patients may represent a clinical phenotype of patients who benefit more than others from early renal support, also because the timing of kidney injury might be often known (e.g., onset of an acute decompensated heart failure or start of cardiopulmonary bypass). Fluid balance is a key issue in these patients (21) due to its immediate detrimental effects of systo-diastolic heart function and ventriculo-arterial coupling, especially in ischemic myocardia after reperfusion (22). Inflammation and a reduction of renal function associated with extracorporeal cardiopulmonary bypass may contribute to multi-organ failure and (even limited) clearance of inflammatory mediators through extracorporeal blood purification may play a clinical role in these patients (23). Finally, cardiac surgery patients typically are carefully monitored in cardiac surgery intensive care units and generally cardiac intensivist are skilled operators regarding extracorporeal circulations (24). All these aspects must be taken into account when analyzing the concept of pre-emptive KST, that cannot, hence, be necessarily extended to other settings (e.g., abdominal sepsis or major non-cardiac surgery).
A final aspect needs to be remarked upon this study: the message coming from the authors seems to focus on the timing of the treatment rather than on its prescription (chosen modality and dialytic dose), anticoagulation strategy, materials (type of membranes), or its direct clinical effects (solute control). However, according to authors’ protocol, KST was prescribed with very specific clinical targets: (I) solute control: (i) BUN ≤30 mmol/L, (ii) RRT dose 25–30 mL/kg/h; (II) volume control: (i) 24 h output ≥ input, (ii) reduction of peripheral edema; (III) metabolism control: (i) 3.5< K+ ≤5.5 mmol/L, (ii) 135< Na+ ≤145 mmol/L, (iii) pH ≥7.25, (iv) HCO3- ≥16 mmol/L, (v) lactic acid normal or near normal; (IV) hemodynamics: (i) MAP ≥65 mmHg, (ii) CVP 8–12 mmHg. We do not have any information on how efficiently and quickly these important targets were ultimately achieved. It is clear that, currently, even if the timing of KST may play a crucial role in specific cohorts of critically ill patients, the other components of the dialytic treatment should also be evaluated in the context of a personalized therapy (25). Not all patients may primarily require early fluid balance adjustment, as in the case of cardiac surgery patients (19,26); equally not all of them may have acid base or electrolyte derangements requiring fast correction, as showed by the Initiation of Dialysis Early Versus Delayed in the Intensive Care Unit (IDEAL-ICU) trial, conducted in septic patients (20). In all cases, clinicians should carefully check several quality indicators of dialysis delivery in order to appraise, in the complex scenario of KST, how to optimize the treatments in each patient (27). Finally, RRT complications (e.g., bleeding in heparin-based anticoagulation strategies, metabolic alterations in citrate-based anticoagulation strategies) and undesired conditions (immobility limiting physiotherapy and physical recovery) have not been reported by the authors. All these negative aspects associated with RRT should be considered when the clinicians are deciding whether anticipate RRT over the classical urgent/emergent indications and carefully weighed against the above listed potential benefits.
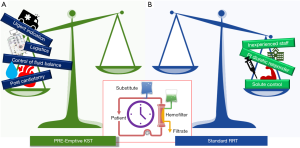
In conclusion, timing is one of the fundamental aspects that has to be identified in order to deliver effective KST. It is possible that an early approach is indicated in a specific setting of patients whereas other indications or clinical settings, according to what currently described by recent clinical trials, may not encourage to anticipate the dialytic treatment (Figure 1). The paper by Guo-Wei Tu and collaborators added some further information regarding the benefit of proactive KST in the specific clinical setting of post cardiotomy patients with cardiogenic shock.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform (available at http://dx.doi.org/10.21037/atm.2020.03.96) The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ricci Z, Romagnoli S, Ronco C. Renal support. Minerva Anestesiol 2011;77:1204-15. [PubMed]
- Forni LG, Darmon M, Schetz M. Renal replacement in 2050: from renal support to renal replacement? Intensive Care Med 2017;43:1044-7. [Crossref] [PubMed]
- Murugan R, Balakumar V, Kerti SJ, et al. Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care 2018;22:223. [Crossref] [PubMed]
- Garzotto F, Ostermann M, Martín-Langerwerf D, et al. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in critically ill patients. Crit Care 2016;20:196. [Crossref] [PubMed]
- Zhu R, Allingstrup MJ, Perner A, et al. The Effect of IV Amino Acid Supplementation on Mortality in ICU Patients May Be Dependent on Kidney Function: Post Hoc Subgroup Analyses of a Multicenter Randomized Trial. Crit Care Med 2018;46:1293-301. [Crossref] [PubMed]
- Girardot T, Schneider A, Rimmelé T. Blood Purification Techniques for Sepsis and Septic AKI. Semin Nephrol 2019;39:505-14. [Crossref] [PubMed]
- Honore PM, Hoste E, Molnár Z, et al. Cytokine removal in human septic shock: Where are we and where are we going? Ann Intensive Care 2019;9:56. [Crossref] [PubMed]
- Tu GW, Xu JR, Liu L, et al. Preemptive renal replacement therapy in post-cardiotomy cardiogenic shock patients: a historically controlled cohort study. Ann Transl Med 2019;7:534. [Crossref] [PubMed]
- Yang XM, Tu GW, Gao J, et al. A comparison of preemptive versus standard renal replacement therapy for acute kidney injury after cardiac surgery. J Surg Res 2016;204:205-12. [Crossref] [PubMed]
- Ronco C, Ricci Z, Husain-Syed F. From Multiple Organ Support Therapy to Extracorporeal Organ Support in Critically Ill Patients. Blood Purif 2019;48:99-105. [Crossref] [PubMed]
- Romagnoli S, Ricci Z. When to start a renal replacement therapy in acute kidney injury (AKI) patients: many irons in the fire. Ann Transl Med 2016;4:355. [Crossref] [PubMed]
- Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, Joannidis M. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med 2018;44:323-36. [Crossref] [PubMed]
- Rewa OG, Bagshaw SM, Wang X, et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: A multicenter, prospective, observational study. J Crit Care 2019;52:109-14. [Crossref] [PubMed]
- Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care 2018;22:101. [Crossref] [PubMed]
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014;10:37-47. [Crossref] [PubMed]
- Cruces P, Lillo P, Salas C, et al. Renal Decapsulation Prevents Intrinsic Renal Compartment Syndrome in Ischemia-Reperfusion-Induced Acute Kidney Injury: A Physiologic Approach. Crit Care Med 2018;46:216-22. [Crossref] [PubMed]
- Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 2015;88:897-904. [Crossref] [PubMed]
- Gaudry S, Hajage D, Schortgen F, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 2016;375:122-33. [Crossref] [PubMed]
- Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney 26. Injury: The ELAIN Randomized Clinical Trial. JAMA 2016;315:2190-9. [Crossref] [PubMed]
- Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med 2018;379:1431-42. [Crossref] [PubMed]
- Haase-Fielitz A, Haase M, Bellomo R, et al. Perioperative Hemodynamic Instability and Fluid Overload are Associated with Increasing Acute Kidney Injury Severity and Worse Outcome after Cardiac Surgery. Blood Purif 2017;43:298-308. [Crossref] [PubMed]
- Trambaiolo P, Bertini P, Borrelli N, et al. Evaluation of ventriculo-arterial coupling in ST elevation myocardial infarction with left ventricular dysfunction treated with levosimendan. Int J Cardiol 2019;288:1-4. [Crossref] [PubMed]
- Lannemyr L, Bragadottir G, Krumbholz V, et al. Effects of Cardiopulmonary Bypass on Renal Perfusion, Filtration, and Oxygenation in Patients Undergoing Cardiac Surgery. Anesthesiology 2017;126:205-13. [Crossref] [PubMed]
- Ricci Z, Benelli S, Barbarigo F, et al. Nursing procedures during continuous renal replacement therapies: a national survey. Heart Lung Vessel 2015;7:224-30. [PubMed]
- Villa G, Ricci Z, Romagnoli S, Ronco C. Multidimensional Approach to Adequacy of Renal Replacement Therapy in Acute Kidney Injury. Contrib Nephrol 2016;187:94-105. [PubMed]
- Zou H, Hong Q, Xu G. Early versus late initiation of renal replacement therapy impacts mortality in patients with acute kidney injury post cardiac surgery: a meta-analysis. Crit Care 2017;21:150. [Crossref] [PubMed]
- Rewa OG, Tolwani A, Mottes T, et al. Quality of care and safety measures of acute renal replacement therapy: Workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care 2019;54:52-7. [Crossref] [PubMed]


