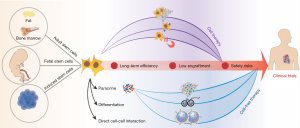
Engineering better stem cell therapies for treating heart diseases
Introduction
Stem cells are a group of primitive cells with the potential of self-replication and multi-directional differentiation (1). Under certain conditions, they can differentiate into multiple adult cells in the body (1). Stem cell therapy, also known as stem cell transplantation, is the delivery of stem cells to a specific part of the body by systemic or local injection to repair diseased or damaged tissue (2). Many human diseases are caused by abnormal lesions or tissue death within certain organs. By transplanting stem cells into these damaged regions, healthy cells can be regenerated, improving organ function and reversing diseased states (3).
For decades, stem cells and their byproducts have shown efficacy in repairing tissues and organs in numerous pre-clinical studies and clinical trials, providing hope for alternate therapies and possible cures for important diseases such as metabolic diseases (4), nervous system diseases (5), blood system diseases (6), autoimmune diseases (7), and cardiovascular diseases (8), including heart disease (9).
Stem cell therapy in heart repair
According to the recent report of American Heart Association, cardiovascular disease is still the number one cause of death worldwide (10). Coronary heart disease, dilated cardiomyopathy, and severe valvular disease can lead to heart failure (HF) due to ischemic necrosis of cardiomyocytes at the end of the disease period (11). At the same time, heart transplantation has problems such as the lack of donors, the need for long-term use of anti-rejection drugs, and high medical expenses.
Since existing treatments still have limited ability to reverse the HF process after myocardial infarction, regeneration of cardiomyocytes has become the direction of many scientists' research. An increasing number of stem cell types have been demonstrated to be visible in cardiac repair, including skeletal muscle progenitor cells, bone marrow stem cells [mesenchymal stem cells (MSCs), hematopoietic stem cell (HSCs), monocytes, etc.], adipose-derived stem cells, bone marrow and blood-derived endothelial progenitor cells, cardiac stromal cells (CSCs), etc. (12).
In August 2016, the biotech company CardioCell announced effective results in the application of stem cells for the treatment of chronic HF indications at the European Society of Cardiology Congress. This was the world’s first phase 2a clinical trial to study the effects of intravenous ischemic tolerance to mesenchymal stem cells (itMSCs) in the treatment of chronic HF (13). The result of this trial turned out to be safe and well-tolerated, but only with marginal efficacy. During the same year, at the annual meeting of the Society for Cardiovascular Angiography and Interventions (SCAI), a number of professors and experts jointly announced promising results for the RENEW (Efficacy and Safety of Targeted Intramyocardial Delivery of Auto CD34+ Stem Cells for Improving Exercise Capacity in Subjects With Refractory Angina) trial (14) and the ATHENA (Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction) trial (15). Although the results of these trials did not sufficiently show significant efficiency due to the early termination (14) and limited sample size (15), they could still be promising development demonstrating the potential for viable stem cell-based heart therapies.
Current limitations and challenges from bench to bedside
Stem cell transplantation has great potential. In theory, stem cells can be differentiated into almost all types of human cells. However, according to the International Society for Stem Cell Research, stem cell transplantation is currently recognized as safe and effective only in the treatment of hematopoietic systems (16). Other widely used stem cell therapies are applied to the skin (in the case of burns) (17), bone (18) and corneal diseases (19), and bone-marrow transplantations (16).
For decades, stem cells have been widely studied in preclinical animal models and clinical trials. However, few of the trials have been approved by the FDA and successfully reached the market. For heart disease like cardiovascular ischemia, stem cell therapies are making headway in clinical trials but have not yet reached the clinic (Table 1). Other cell-based therapies for HF or cardiomyopathy include DREAM-HF (phase 3 trial of mesenchymal precursor cells in chronic HF) (20), CONCERT-HF (combination of mesenchymal and c-kit+ cardiac stem cells as regenerative therapy for heart failure) (21), ELPIS (allogeneic human MSC injection in patients with hypoplastic left heart syndrome) (22), and POSEIDON-DCM (comparison of allogeneic vs. autologous MSCs for non-ischemic dilated cardiomyopathy) (23). These ongoing trials are expected to have available results in 2020.
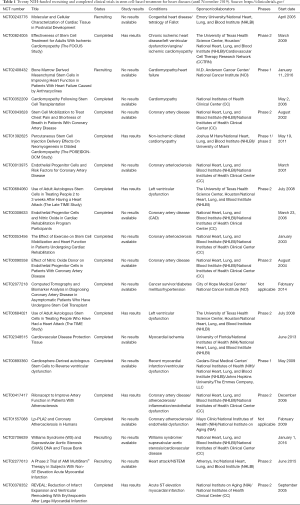
Full table
Increasing research on stem cell therapies for acute myocardial infarction (AMI) has put in doubt the traditional notion that the heart cannot be repaired. By extension, the enthusiasm for stem cell therapies that target cardiovascular disease continues to rise. But unfortunately, either the pre-clinical research of stem cell-based cardiac regeneration or clinical trials of stem cell therapies still have a number of limitations (24). Why does this advanced therapeutic option encounter barriers before being able to benefit the public, and what are the challenges that the scientific community must overcome before implementation is possible?
Long-term efficacy
Despite evidence of short-term improvements in heart performance, it is not clear whether heart stem cell therapies have long-term benefits. In April 2009, Meyer et al. published a long-term (5-year) follow-up of a clinical trial involving bone marrow cell transplantation to promote ST-segment elevation myocardial infarction regeneration (BOOST) (25). The results showed that left ventricular function, measured by left ventricular ejection fraction (LVEF), was significantly improved compared with the control group after 6 months. However, there was no significant difference in improvement in left ventricular cardiac function or major adverse cardiovascular events (MACEs) between the two groups long-term follow-up at 5 years after the treatment was applied. The investigators believed that despite the faster recovery of LVEF in the treatment group, the lack of long-term improvement of left ventricular systolic function in AMI patients who received stem cell transplantation needs to be addressed (25).
Uncontrollable biodistribution
The poor engraftment of stem cells at the site of injury or disease is considered to be a primary explanation for the low efficacy of some stem cell trials (26,27). The traditional systemic delivery of stem cells, accomplished through intravenous injection, while facile, is not particularly good at getting cells where they need to be. What’s more, a larger portion of the injected cells accumulate in other organs, such as the lungs (28). One alternative method is to directly inject cells or byproducts into the injury tissue. This has been a popular research strategy for heart repair. We and many others usually administer therapeutic stem cells into the infarct border zone of the heart via intramyocardial injections (29,30). An obvious shortcoming of this method is that it generally requires an open-chest surgery, leading to increased post-operative pain and general risk to the patient.
Another clinical obstacle that must be addressed is the low survival rate of stem cells in vivo (26). In many of the clinical trials of stem cell-based heart repair, autologous cells are intravenously or intracoronarily injected into the patient (31). Somehow, after 24 to 48 hours of transplantation, usually only a small fraction of cells (about 5%) remain in the transplanting site. Four to six weeks after transplantation, 99% of the retained cells do not survive (31). One of the reasons believed to cause the diminished viability of the cells is the harsh environment in the heart or other organs, which threatens their proliferation, accelerating apoptosis and migration to other issues (26).
Risk of tumorigenicity and immunogenicity
In May 2001, an Israeli nine-year old boy was diagnosed with ataxia-telangiectasia, a rare neurological disease that unfortunately has no treatment. He received embryonic stem cell injection in his brain in Moscow with the last remaining hope of improving his condition. Various regions of his brain were injected with the embryonic cells. Four years later, tumors were found in his brain. And two embryonic stem cells were detected among the tumor cells (32). This story, which is the first-reported case of stem cell therapy causing a brain tumor, engendered a rejection to stem cell treatment by the local people. Fortunately, the tumor was diagnosed to be benign and safely removed.
The risk of tumorigenicity, remaining a terrifying concern for the public, limits their acceptance to stem cell-based therapy. The concern is not unwarranted either. Stem cells are biologically similar to tumor cells in many respects (33). They exhibit sustained proliferation, insensitivity to apoptosis, and similar growth regulation mechanisms as tumor cells. It has been found from animal models that human embryonic stem cells or induced pluripotent stem cells can cause both benign teratomas and malignant teratomas (33). Their pluripotency is considered to be the biological basis of tumor formation. Understanding this biological basis better and more fully is key to preventing future cases of tumor formation, as illustrated by the young patient’s case above.
Host immunity is a serious challenge to consider when injecting non-autologous cells or agents into patients. On the other hand, autologous products do not risk immune rejection, but must be collected from the patient and expanded/manipulated before infusing back into the patient. The collection of cells, which usually requires a biopsy, from already diseased patients presents an added health risk.
Mechanisms of stem cell-based therapy for heart repair
In order to address the safety and efficacy issues faced by cell-based cardiac therapies, we need to firstly elucidate the therapeutic mechanisms involved. According to FDA regulations, every drug comes into the market after its therapeutic mechanisms and safety profiles have been widely accepted (34). However, with stem cells, the therapeutic mechanisms are illusive; even more so when it comes to their application for the treatment of heart diseases. In other words, transplanted cells may have unpredictable and uncontrollable behaviors in tissues/organs, such as heart, as a result of their developmental pluripotency. Popular and promising as they are, stem cell therapies still lack elucidation. Among the many questions left to answer are: how do they move in vivo? Where do they go? Why do they behave the way they do?
Initially, the stem cell therapy field had two schools of thought when it comes to the treatment of heart disease. One is the “replacement” theory. In this scenario, transplanted stem cells differentiate into cardiomyocytes, replacing the cells that were lost due to myocardial infarction (35). Patients can lose up to half billion cardiomyocytes in a major heart attack event. Studies on the differentiation from stem cells to cardiomyocytes have been accumulating since the theory was first established (36). However, the low survival rates and engraftment efficiency recorded in many studies have put into question the importance of this mechanism. Meanwhile, a number of studies have shown that adult stem cells can not differentiate into cardiomyocytes, but this seems not affect their ability of repairing damaged myocardium, and improving myocardial function (2). An ever-improving array of detection techniques continue increase the odds that the mechanisms behind their behavior will be understood.
The other point of view is the “awake” theory, in which stem cells secrete cytokine nutrients, promote endogenous cell proliferation, and thereby reduce the number of cells that are dying due to myocardial infarction (2). Paracrine activity is an important process for cells to communicate with other nearby cells. It appears to be especially valuable for active intercellular process in the body such as stem cell-based regeneration (37). Indeed, there is now a large body of evidence supporting the hypothesis that paracrine mechanisms are crucial for tissue regeneration (37-39). In recent years, more evidence suggests that transplanted stem cells exert their therapeutic effects by secreting biologically active proteins, or paracrine factors, to resident cells (39). In the heart, there are also various types of paracrine factors playing key roles in cardiac repair, including growth factors and chemokines, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), and secreted frizzled related protein 2 (Sfrp2) (37,39).
The heightened interest in paracrine signals has spurred the increased focus on extracellular vesicles (EVs) transporting those molecules and a move away from the use of cells themselves. The most popular of these vesicles is the exosome, which is also the means by which most of the molecules are transmitted (40). Exosomes, as the functional paracrine units of therapeutic cells, can partially reproduce the reparative properties of their parental cells (41,42). Exosomes have become a popular research focus for us and many others in the past five years because of their primary role in cell-cell communication, including stem cell-derived exosome’s therapeutic role in heart repair (41). The cargo carried by these exosomes, as well as the membrane proteins that characterize them vary by cell type and cellular microenvironment (40). Our lab has isolated exosomes from explant-derived CSCs sourced from patients with HF (FEXO) or from normal (non-diseased) donor hearts (NEXO) and compared their regenerative activities in vitro and in vivo (43). The results suggest that the HF altered the miRNA cargos of cardiac-derived exosomes and impaired their regenerative activities. We demonstrated that miR-21-5p contributes to exosome-mediated heart repair by enhancing angiogenesis and cardiomyocyte survival through the phosphatase and tensin homolog/Akt pathway. Many of these experiments were conducted using conditioned media, which is the media that nourishes the cell in-vitro, absorbing the proteomic and exosomal output that the cells release after a number of days. When conditioned media was injected into infarcted cardiac tissue, reparative and regenerative effects comparable to direct cell transplantation where observed.
Surprisingly, there is another important intracellular communication method that is likely to be ignored by many studies in the past few years. Direct cell-cell interaction has been reported to be crucial to the functional regeneration of stem cell therapies (44-46). As early as 2003, Fukuhara et al. has co-cultured bone marrow stromal cells (BMCs) with cardiomyocytes and found that direct cell-cell contact with cardiomyocytes was important for BMCs to trigger some potential environmental factors of differentiation in vitro (44). Later on, in 2017, when some researchers co-transplanted MSCs and HSCs to MI mice heart, their results demonstrated that mechanism of HSCs promoting cardiac regeneration lay in their angiogenesis ability. Meanwhile, transplanted MSCs showed the capability for intercellular communication with surrounding cardiomyocytes by gap junctional signaling (45). However, in-depth in vivo studies are still needed to furtherly confirm the importance of direct cell-cell crosstalk in stem cell-based cardiac remodeling. Specifically, in vivo gap junction blocking approach, combined with in vitro cell co-culture may give us a better understanding of the interaction process between transplanted stem cells and neighboring cardiac cells.
New era: bioengineering strategies
Bioengineers have been able to utilize the limited mechanistic information available to develop advanced therapeutic strategies using stem cells. Engineering methods that aim to realize the multifunctionalization of stem cell therapies have been thriving in the past five years (47). With the collaboration of physicians, chemists, and biologists, bioengineers are able to develop stem cell-based therapies that combine stem cells, or their byproducts, with biomaterials in order to enhance therapeutic efficiency.
Improving targeting ability
The first challenge stem cell therapy faces is the effective delivery. To be specific, similar to regular drug-based treatment, it is important to send cells to the injury site in a targeted manner. Achieving this is one of the goals of bioengineering. Previously, our lab has used FDA-approved ferumoxytol nanoparticles to attempt magnetic targeting in the body (48,49). As a technique continuously improved in this field, an externally introduced magnetic field was set up near the injury spot in the heart. During the injection of iron-labelled (ferumoxytol) stem cells, the magnetic field attracting them directly to the injured cardiac tissue. However, the use of a strong magnetic field during an operative procedure may have unexpected consequences on the equipment as well as the patient. The development of a more biosafe targeting strategy was needed.
Thus, we began to focus on the platelet, a unique component in blood which can also accumulate and bind directly to injured endothelial cells on blood vessels. Previously, our group has developed an innovative method to decorate the surface membrane of CSCs with platelet nanovesicles (PNVs) (50). Our engineered PNV-fused CSCs were demonstrated to express platelet surface markers that are associated with platelet adhesion to injury sites, enhancing the targeted vascular delivery of CSCs to the site of myocardial infarction.
In addition, platelet membranes indicate an alternative solution to adhere injected stem cells to the injured endothelium (51). Recently, our group successfully synthesized a platelet-inspired nanocell (PINC) that incorporates both prostaglandin E2 (PGE2)-modified platelet membrane and cardiac stromal cell-secreted factors (43). The natural infarct-homing ability of platelet membranes and the overexpression of PGE2 receptors in the injury microenvironment of heart after myocardial ischemia/reperfusion, gave us the inspiration to design this unique combo. Our PINCs have been demonstrated to achieve the targeted delivery of therapeutic payloads to the injured cardiac tissue.
Moreover, platelets can be functionalized on their membrane which generates another promising solution for targeted delivery. Those more specific units are antibodies. Studies from our lab and others have also demonstrated that antibodies against biomarkers that specifically express under heart ischemic diseases, such as CD34, can serve as a targeted mediator, not only navigating transplanted stem cells to the injured heart, but recruiting circulating endogenous stem cells to the ischemic site (52,53). Specifically, taking advantage of the natural infarct-homing ability of platelets and their ability to bind to circulating CD34+ progenitors in patients and improve prognosis, we engineered CD34 antibody-linked platelets (P-CD34) to capture circulating CD34-positive endogenous stem cells and direct them to the site of myocardial infarction (53). Similarly, CD41 antibodies, binding to platelets, can also be used to target the MI area. Taking advantage of pre-targeting and bioorthogonal chemistry (PTBC), we engineered a PTBC system using bioorthogonal click reaction to link these two antibodies (CD34 and CD41) in vivo, engaging endogenous stem cells with circulating platelets (54). As a result, the platelets redirected the stem cells to the injured cardiac tissue and enhanced repairing efficiency.
Bispecific antibodies (BsAbs), promising therapeutic agents used in cancer immunotherapy, can also be utilized to treat cardiovascular diseases. In the most recent study, our lab designed BsAbs by the chemical cycloaddition of F(ab')2 fragments from monoclonal anti-CD34 and anti-cardiac myosin heavy chain (CMHC) antibodies, which specifically target circulating CD34-positive cells and injured cardiomyocytes simultaneously (55). However, the major disadvantage of antibody-based targeting is that some particular biomarkers are only expressed during AMI.
Overcoming low cell retention
After achieving more efficient targeted delivery of the cellular therapy, the next obstacle to overcome is improving cell engraftment in the injury site. To improve the low retention and survival rates of transplanted stem cells, many innovative biomaterials have been developed in the past decade that encapsulate them and protect them once injected. Injectable hydrogels have been designed with different types of materials and combined with particular stem cells inside. Previously, our lab demonstrated the safety and efficacy of encapsulating CSCs in thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) or P(NIPAM-AA) nanogels in mouse and pig models of myocardial infarction (MI) (56). In a recent study, we created a hydrophilic and negatively charged microenvironment using poly(N-isopropylacrylamide-co-itaconic acid), which is favorable for maintaining high viability of CSCs (57). The results revealed the treatment promoted MI heart repair through angiogenesis and inhibition of apoptosis with an improved cell retention rate. What is more, the other advantage of many hydrogels is that they do not elicit systemic inflammation or local T cell infiltrations in immunocompetent mouse models.
In addition to the idea of hydrogels, another revolutionary biomaterial used in the area of cardiac regeneration is the cardiac patch. The delivery of therapeutic cells in a cardiac patch increases cell retention and represents other functionalization aims. We have reported a novel strategy for creating a vascularized cardiac patch featuring biomimetic microvessels (BMVs) in a fibrin gel and spiked with human CSCs (58). Our results showed that the endothelialized BMVs could mimic the natural architecture and function of capillaries and that the vascularized cardiac patches (BMV-CSC patch) have great regenerative potential. Meanwhile, a shortcoming of the epicardial patches remains their slow integration with host myocardium. To address this issue, our group engineered a microneedle patch integrated with cardiac stromal cells (MN-CSCs), utilizing polymeric MNs to create communication channels between host myocardium and therapeutic CSCs (59).
The cardiac patch strategies normally require open-chest surgery, which is risky for the patient and requires a long recovery. In comparison, hydrogels can be injected directly to the site of injury using a minimally-invasive operation. Ventrix, a subsidiary of the University of California, San Diego, completed the first successful, minimally invasive human trial using a heart-repairing hydrogel approved by the FDA (60). The trial was the first to test a hydrogel used to repair heart tissue and also the first to test a hydrogel made from the natural scaffold of myocardial tissue, also known as extracellular matrix (ECM). Results have shown that this hydrogel, called VentriGel, can be safely injected through a catheter into patients who have had a heart attack in the past 2 to 36 months (60). Once injected into the damaged myocardium, VentriGel forms a scaffold that creates a healing environment for healthy cell migration, promoting new cardiac tissue formation. Ventrix is currently preparing for phase II clinical trials (60).
Cell-free therapy
An unavoidable risk with stem cell transplantations is that of tumorigenicity or immunogenicity, as we discussed above. Thus, many in the field have gravitated toward the study of bioactive agents released from stem cells, which have had comparable therapeutic effects, suggesting the possibility of a promising alternative to stem cell therapies. The most important bioactive agents currently being studied are EVs, including microvesicles and exosomes, which contain the biologically active components [mRNA, miRNAs, proteins (growth factors)] found in stem cells. These have been shown to have salutary effects (similar to cell therapies) on myocardial repair after injury. Compared to other EVs, exosomes have been more widely regarded as candidates for cell-free therapy and have been tested by our lab and others in the treatment of pulmonary fibrosis, cancer therapy, myocardial infarction, etc. For example, our lab has found that exosomes obtained from atorvastatin-pretreated MSCs have significantly enhanced therapeutic efficacy in treating MI (9). Nevertheless, many preclinical or clinical protocols for the application of exosomes have not been standardized, including their extraction and purification. Thus, much work is left to be done before exosomes are successful in clinical trials.
Biomimetic strategies in stem cell-based therapy
While the therapeutic strategies discussed above have commonly focused on the functionalization of stem cells, there is another groundbreaking innovation based on the creation of ‘super stem cells’, by which we mean the construction of synthetic stem cells or cell-mimicking composites (61). This idea has recently been attempted twice by our group, for the treatment of heart diseases. For the first time, we reported a ‘core-shell’ design for a therapeutic microparticle (MP) which mimicked stem cell biointerfacing during regeneration (62). Named cell-mimicking MP (CMMP), this artificial stem cell contained control-released stem cell factors in its polymeric core and was cloaked with stem cell membrane fragments on the surface. In our mouse model of myocardial infarction, injection of CMMPs resulted in a similar augmentation of cardiac functions in comparison to direct CSC therapy. What is more important, CMMPs did not stimulate T cell infiltration in immuno-competent mice, suggesting their great potential for clinical trials. Subsequently, we sought to create a more complex stem cell-mimicking composite. We successfully packaged secreted factors from human bone marrow-derived MSC into Poly(lactic-co-glycolic acid) PLGA microparticles and then coated them with MSC membranes (63). These therapeutic particles, “synthetic MSC” (or synMSC), demonstrated their regenerative potential in mice with AMI.
Conclusions
Throughout decades of stem cell pre-clinical studies and clinical trials, challenges and risks exist, also, prospects and innovations exist. How to overcome the most conspicuous shortcomings with more innovative strategies is a question for every bioengineer, which has been trying to do and needs to be done in the future (Figure 1). In any case, an indispensable premise is a more comprehensive interpretation and understanding of the mechanism under which stem cells benefit cardiac regeneration. As we mentioned before, in addition to the paracrine effect, we still believe that direct cell-cell contact plays a vital role in this process. Therefore, the combination of the paracrine effect and the potential activation of the intrinsic program of cardiac cells, which is triggered by cell-cell crosstalk, followed by further observation of cell fate, cell niche, and cell in situ migration, are our top priorities for the next decade of advancing stem cell therapy for heart repair.
Acknowledgments
Final grammatical edits were completed by Jhon Cores, PhD.
Funding: This work was supported by grants from the National Institutes of Health (R01 HL123920, HL137093, HL144002, HL146153, and HL147357 to K Cheng) and the American Heart Association (18TPA34230092 and 19EIA34660286 to K Cheng).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.44). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Körbling M, Estrov Z. Adult stem cells for tissue repair—a new therapeutic concept? N Engl J Med 2003;349:570-82. [Crossref] [PubMed]
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 2008;451:937. [Crossref] [PubMed]
- Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol 2016;2016:6940283.
- Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 2010;6:195-213. [Crossref] [PubMed]
- Volkman R, Offen D. Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 2017;35:1867-80. [Crossref] [PubMed]
- Bordignon C. Stem-cell therapies for blood diseases. Nature 2006;441:1100. [Crossref] [PubMed]
- Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009;27:2624-35. [Crossref] [PubMed]
- Majka M, Sulkowski M, Badyra B, et al. Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl Med 2017;6:1859-67. [Crossref] [PubMed]
- Huang K, Hu S, Cheng K. A New Era of Cardiac Cell Therapy: Opportunities and Challenges. Adv Healthc Mater 2019;8:e1801011. [PubMed]
- Benjamin EJ, Muntner P, Bittencourt MS. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56-e528. [Crossref] [PubMed]
- Elgendy IY, Mahtta D, Pepine CJ. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ Res 2019;124:1520-35. [Crossref] [PubMed]
- Nguyen PK, Rhee JW, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol 2016;1:831-41. [Crossref] [PubMed]
- Butler J, Epstein SE, Greene SJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II-A randomized trial. Circ Res 2017;120:332-40. [Crossref] [PubMed]
- Povsic TJ, Henry TD, Traverse JH, et al. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. JACC Cardiovasc Interv 2016;9:1576-85. [Crossref] [PubMed]
- Henry TD, Pepine CJ, Lambert CR, et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Interv 2017;89:169-77. [Crossref] [PubMed]
- Hyun I, Lindvall O, Ahrlund-Richter L, et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell 2008;3:607-9. [Crossref] [PubMed]
- Fu X, Li H. Mesenchymal stem cells and skin wound repair and regeneration: possibilities and questions. Cell Tissue Res 2009;335:317-21. [Crossref] [PubMed]
- Lee KB, Hui JH, Song IC, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem cells 2007;25:2964-71. [Crossref] [PubMed]
- Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55. [Crossref] [PubMed]
- Borow KM, Yaroshinsky A, Greenberg B, et al. Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ Res 2019;125:265-81. [Crossref] [PubMed]
- Bolli R, Hare JM, March KL, et al. Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit(+) Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ Res 2018;122:1703-15. [Crossref] [PubMed]
- Kaushal S, Wehman B, Pietris N, et al. Study design and rationale for ELPIS: A phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am Heart J 2017;192:48-56. [Crossref] [PubMed]
- Hare JM, DiFede DL, Rieger AC, et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol 2017;69:526-37. [Crossref] [PubMed]
- Behfar A, Crespo-Diaz R, Terzic A, et al. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol 2014;11:232-46. [Crossref] [PubMed]
- Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J 2009;30:2978-84. [Crossref] [PubMed]
- Heslop JA, Hammond TG, Santeramo I, et al. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med 2015;4:389-400. [Crossref] [PubMed]
- Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810-34. [Crossref] [PubMed]
- Jung JW, Kwon M, Choi JC, et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J 2013;54:1293-6. [Crossref] [PubMed]
- Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011;108:792-6. [Crossref] [PubMed]
- Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res 2010;106:1570-81. [Crossref] [PubMed]
- Nguyen PK, Neofytou E, Rhee JW, et al. Potential Strategies to Address the Major Clinical Barriers Facing Stem Cell Regenerative Therapy for Cardiovascular Disease: A Review. JAMA Cardiol 2016;1:953-62. [Crossref] [PubMed]
- Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 2009;6:e1000029. [Crossref] [PubMed]
- Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998. [Crossref] [PubMed]
- Overington JP, Al-Lazikani B, Hopkins AL. Opinion - How many drug targets are there? Nat Rev Drug Discov 2006;5:993-6. [Crossref] [PubMed]
- Boheler KR, Czyz J, Tweedie D, et al. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002;91:189-201. [Crossref] [PubMed]
- Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012;111:344-58. [Crossref] [PubMed]
- Gnecchi M, Zhang ZP, Ni AG, et al. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ Res 2008;103:1204-19. [Crossref] [PubMed]
- Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 2006;20:661-9. [Crossref] [PubMed]
- Hodgkinson CP, Bareja A, Gomez JA, et al. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res 2016;118:95-107. [Crossref] [PubMed]
- Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 2014;23:1045-59. [Crossref] [PubMed]
- Kishore R, Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res 2016;118:330-43. [Crossref] [PubMed]
- Vandergriff AC, de Andrade JB, Tang J, et al. Intravenous Cardiac Stem Cell-Derived Exosomes Ameliorate Cardiac Dysfunction in Doxorubicin Induced Dilated Cardiomyopathy. Stem Cells Int 2015;2015:960926.
- Qiao L, Hu S, Liu S, et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest 2019;129:2237-50. [Crossref] [PubMed]
- Fukuhara S, Tomita S, Yamashiro S, et al. Direct cell-cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg 2003;125:1470-9. [Crossref] [PubMed]
- Lemcke H, Gaebel R, Skorska A, et al. Mechanisms of stem cell based cardiac repair-gap junctional signaling promotes the cardiac lineage specification of mesenchymal stem cells. Sci Rep 2017;7:9755. [Crossref] [PubMed]
- Xie Y, Ibrahim A, Cheng K, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells 2014;32:2397-406. [Crossref] [PubMed]
- Li Z, Hu S, Cheng K. Chemical Engineering of Cell Therapy for Heart Diseases. Acc Chem Res 2019;52:1687-96. [Crossref] [PubMed]
- Vandergriff AC, Hensley TM, Henry ET, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014;35:8528-39. [Crossref] [PubMed]
- Cores J, Caranasos TG, Cheng K. Magnetically Targeted Stem Cell Delivery for Regenerative Medicine. J Funct Biomater 2015;6:526-46. [Crossref] [PubMed]
- Tang J, Su T, Huang K, et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng 2018;2:17-26. [Crossref] [PubMed]
- Hu Q, Sun W, Wang J, et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng 2018;2:831-40. [Crossref] [PubMed]
- Lee RJ, Fang Q, Davol PA, et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cells 2007;25:712-7. [Crossref] [PubMed]
- Shen D, Li Z, Hu S, et al. Antibody-Armed Platelets for the Regenerative Targeting of Endogenous Stem Cells. Nano Lett 2019;19:1883-91. [Crossref] [PubMed]
- Li Z, Shen D, Hu S, et al. Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair. ACS Nano 2018;12:12193-200. [Crossref] [PubMed]
- Huang K, Li Z, Su T, et al. Bispecific Antibody Therapy: Bispecific Antibody Therapy for Effective Cardiac Repair through Redirection of Endogenous Stem Cells (Adv. Therap. 10/2019). Advanced Therapeutics. 2019;31. [Crossref]
- Tang J, Cui X, Caranasos TG, et al. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017;11:9738-49. [Crossref] [PubMed]
- Cui X, Tang J, Hartanto Y, et al. NIPAM-based Microgel Microenvironment Regulates the Therapeutic Function of Cardiac Stromal Cells. ACS Appl Mater Interfaces 2018;10:37783-96. [Crossref] [PubMed]
- Su T, Huang K, Daniele MA, et al. Cardiac Stem Cell Patch Integrated with Microengineered Blood Vessels Promotes Cardiomyocyte Proliferation and Neovascularization after Acute Myocardial Infarction. ACS Appl Mater Interfaces 2018;10:33088-96. [Crossref] [PubMed]
- Tang J, Wang J, Huang K, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv 2018;4:eaat9365.
- Traverse JH, Henry TD, Dib N, et al. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl Sci 2019;4:659-69. [Crossref] [PubMed]
- Hu S, Ogle BM, Cheng K. Body builder: from synthetic cells to engineered tissues. Curr Opin Cell Biol 2018;54:37-42. [Crossref] [PubMed]
- Tang J, Shen D, Caranasos TG, et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 2017;8:13724. [Crossref] [PubMed]
- Luo L, Tang J, Nishi K, et al. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ Res 2017;120:1768-75. [Crossref] [PubMed]