
Validation of the prognostic value of various lymph node staging systems for cervical squamous cell carcinoma following radical surgery: a single-center analysis of 3,732 patients
Introduction
Although the incidence and mortality rate of cervical cancer has decreased significantly with increasing use of Pap smear for screening and the development of vaccines, it continues to be the fourth most frequent contributor to cancer-related female mortality around the world, especially in developing countries (1). About 80% of cervical cancers are squamous cell carcinoma (2). For patients with Federation International of Gynecology and Obstetrics (FIGO) stage IB-IIA cervical cancer, radical hysterectomy with pelvic lymphadenectomy (RHPL) is considered the standard surgical treatment (3). However, the 2009 FIGO staging system does not take lymph node (LN) status into account (4), and the TNM pN staging system simply stratifies the LN status into N0 (LN negative) and N1 (LN positive) (5). Therefore, the merits of LN metastasis as a prognostic indicator have long been underestimated by previous cervical cancer staging systems. As its cancer staging system is improving, the FIGO Committee has added IIIC1P (pelvic LN metastasis only) and IIIC2P (para-aortic LN metastasis) to the FIGO 2018 staging system based on the improvements in medical technology worldwide and the effect of LN metastasis on prognosis (6). Nevertheless, this approach neglects the number of involved LN, which may limit the effect of its prognostic significance. Recently, a number of studies have revealed that different parameters of LN status, such as the number of positive LNs (PLN), number of negative LNs (NLN), ratio of involved to removed nodes (LN ratio, LNR), and log odds of positive nodes (LODDS), could indicate prognosis among cancer patients (7-15).
However, the notion that dissecting a larger number of LN can achieve better prognosis is yet to be ascertained, and to date, there have seldom been studies comparing the prognostic value of the LN status in cervical squamous cell carcinoma (CSCC), which is less likely to develop LN metastasis than adenocarcinoma and adenosquamous cell carcinoma (16). The purpose of this study was to carry out evaluation and comparisons of the values of the pN stage, 2018 FIGO Stage, PLN, NLN, LNR, and LODDS in the prognoses of CSCC patients who were initially treated with RHPL at a large cancer institution.
Methods
Patients
We retrospectively included CSCC patients with FIGO [2009] stage IB1-IIa2 who underwent abdominal radical hysterectomy +/- bilateral salpingo-oophorectomy, and pelvic +/− para-aortic lymphadenectomy from 2006 to 2014 at Fudan University Shanghai Cancer Center. All of the patients enrolled had undergone standard pelvic lymphadenectomy by experienced gynecological oncologists, which was reviewed from the electronic medical charts. All nodal and fatty tissues from the furcation level of the common iliac artery upwards to the circumflex vein and above the obturator nerve were taken away. If intraoperative palpation suggested suspected para-aortic LN involvement, or if intraoperative frozen section examination showed positive para-aortic or standard iliac LN, para-aortic LN resection was performed as previously described (17). The review of each microscopic slide was conducted by the same gynecologic pathologist, with a second experienced pathologist carrying out confirmation. Patients who received neoadjuvant chemotherapy or preoperative radiotherapy died within 30 days after surgery and had a follow-up time less than three months were excluded in this study. Our research received approval from the Ethics Committee at Fudan University Shanghai Cancer Center.
Adjuvant therapeutic strategies were carried out adhering to the National Comprehensive Cancer Network (NCCN) guidelines. Individuals who had a minimum of one high-risk factor (parametrial margin invasion, LN metastasis, or positive surgical margins) and those who had two or more intermediate-risk factors (positive lymph-vascular space invasion (LVSI), deep depth of myometrial invasion, or tumor diameter ≥4 cm) received adjuvant radiotherapy or simultaneous chemoradiotherapy. Having just one intermediate-risk factor saw the patient exempted from any adjuvant treatment. Following treatment, followed-up was performed after each three-month period over the first two years, after each six-month period over the next three years, and on a yearly basis following this. In cases where patients received adjuvant therapy, follow-ups were initially conducted every month for a six-month period post-surgery. Follow-up visits included examinations of the pelvis, abdominal ultrasonography, chest X-ray, routine blood test, serum squamous cell carcinoma antigen (SCC-Ag), vaginal cytology, magnetic resonance imaging (MRI), or computed tomography (CT) scan.
Classifications of LN
The numbers of LN removed, and the numbers of PLN were consistently recorded in the medical records of each patient. In this study, we used six different classifications of LN status to evaluate the prognostic meaning further. Tree-based recursive partitioning was used to find out optimal cut-points, respectively:
pN stage
pN stage was evaluated in accordance with nodal staging of the TNM system. N0 was defined as LN negative, while N1 was defined as LN positive by the presence of not less than one positive LN.
2018 FIGO stage
2018 FIGO stage was calculated according to the 2018 FIGO staging system, 2018 FIGO stage 0 was defined as LN negative, 2018 FIGO stage 1 as pelvic LN metastasis only, and 2018 FIGO stage 2 for para-aortic LN metastasis.
PLN
PLN was defined as the number of positive LN, and it was divided into 3 groups: PLN 0 (PLN =0), PLN 1 (1≤ PLN ≤5), and PLN 2 (PLN >5).
NLN
NLN was defined as the number of negative LN, and it was divided into 4 groups: NLN 1 (NLN ≤7), NLN 2 (7< NLN ≤17), NLN 3 (17< NLN ≤22), and NLN 4 (NLN >22).
LNR
LNR was defined as the ratio between the number of PLN and the total number of removed LN, and it was divided into 4 groups: LNR 1 (LNR =0), LNR 2 (0< LNR ≤0.033), LNR 3 (0.033< LNR ≤0.159), and LNR 4 (0.159< LNR ≤1).
LODDS
The calculation of LODDS values was done using empirical logistic formula, log [(PLN +0.5)/(NLN +0.5)]. Its definition was the log of the ratio between the number of PLN and the number of NLN. The addition of 0.5 was made to both the numerator and denomination with the purpose of avoiding singularity. LODDS was divided into 4 groups: LODDS 1 (−1.95< LODDS ≤−1.32), LODDS 2 (−1.32< LODDS ≤−1.00), LODDS 3 (−1.00< LODDS ≤−0.73), and LODDS 4 (−0.73< LODDS ≤1.33).
Statistical analyses
Scatter plots with the Pearson correlation coefficient served as a basis for investigating the correlations between LOODS, LNR, NLN, and PLN. Log-rank analysis aided analysis of correlation between patients' clinicopathological characteristics and five-year progression-free survival (PFS) and overall survival (OS), PFS was considered to be the time from primary surgery until the first disease progression, and OS as the period of time beginning with the date the primary surgery was carried out and ending with death or the latest observation. PFS and OS between different groups of patients were compared by Kaplan-Meier survival analysis. Calculations of hazard ratios (HR) and 95% confidence intervals (CI) were made through univariate and multivariate analyses by means of Cox proportional hazards models to produce an evaluation of the prognostic factors related to survival. We adopted two approaches to conduct accurate evaluation and comparison of the relative discriminative abilities of the various LN staging systems: one founded on the Harrell’s concordance index (C-index) estimate, and the other based on the Akaike’s Information Criterion (AIC). In general, a higher C-index represents a better discrimination ability, and a predictive model with a lower AIC demonstrated a better model adaption. Statistical significance was considered to exist when P<0.05. SPSS (Version 22.0) and R software (Version 3.5.2) carried out all statistical analyses.
Results
Clinical and pathological characteristics and survival analysis
A total of 3,732 stage IB1-IIa2 CSCC patients, with a mean age of 47.15 years (range, 20–97), were deemed eligible for inclusion in this analysis. Among the patients, 194 (5.3%), 128 (3.5%) and 928 (24.9%) cases had a positive parametrial invasion, vaginal margin invasion, and LN metastasis, respectively (Table 1). The mean follow-up period was 39.6 months (range, 4–117 months). Every patient received radical abdominal surgery at our center; 100% of patients received the pelvic lymphadenectomy, and 603 patients (16.16%) underwent the para-aortic lymphadenectomy. There were 2,458 patients (65.86%) receiving postoperative adjuvant therapy, including adjuvant radiotherapy and chemotherapy.
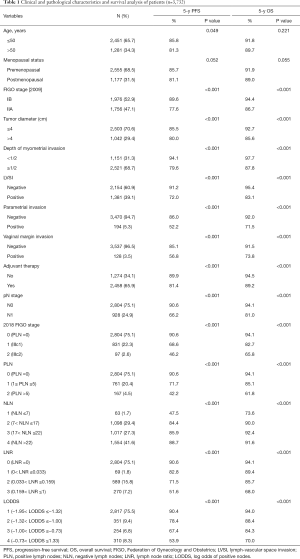
Full table
Among the 3,732 patients, the mean total number of nodes removed was 22.62 (range, 1–70 nodes), and the mean number of positive nodes was 0.95 (range, 0–44 nodes). According to the classification system described in methods, patients were assigned to different groups separately (Table 1). The relationship between clinical and pathologic variables and the 5-year PFS and OS was shown in Table 1. When studying the relationship between different LN staging systems and patients' survival, we found that all of the six LN staging systems were associated strongly with PFS and OS, respectively. The PFS (Figure 1) and OS (Figure 2) for the six LN staging systems were analyzed by the Kaplan-Meier survival curves, respectively.
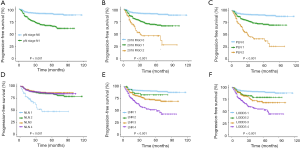

Correlation between PLN, NLN, LNR, and LODDS
Specifically, to understand the relationship between PLN, NLN, LNR, and LODDS, scatter plots were created between any two variables of these classifications. As shown in Figure 3, there was a certain linear correlation between any two variables (all P<0.001). Notably, the value of LODDS and LNR rose as the number of PLN rose (Figure 3A,B), suggesting that LODDS and LNR correlated with PLN. Similarly, LODDS and LNR correlated closely but were not perfectly linear. LNR of between 0.2 and 0.8 saw the LODDS increase much slower and plateau. In contrast, LNR of 0 or 1 resulted in a relationship with increased heterogeneity (Figure 3C), which implies the discriminatory power of the LODDS system may be higher in patients who have either very low or high LNR.
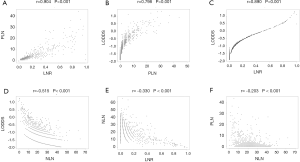
Univariate and multivariate analysis of prognostic factors for PFS and OS
Moreover, Cox regression model was applied to assess the association between clinical and pathologic factors and PFS and OS. The univariable Cox model results showed that all of the six LN staging systems were prognostic indicators for PFS and OS (all P<0.001, Table 2). To avoid multicollinearity in the analysis, we performed multivariate survival analysis with adjustments taking into account significant factors from the univariate analysis of the various models, including only one of these LN staging systems each time. The different models included pN stage (Model 1), 2018 FIGO stage (Model 2), PLN (Model 3), NLN (Model 4), LNR (Model 5), and LODDS (Model 6), separately, and Model 7 for the combination of all the nodal systems. The results showed that pN, 2018 FIGO stage, PLN, LNR, and LODDS were all significant prognostic factors for PFS and OS in Model 1 (PPFS <0.001, POS =0.005), Model 2 (PPFS <0.001, POS =0.003), Model 3 (PPFS <0.001, POS <0.001), Model 5 (PPFS <0.001, POS <0.001), and Model 6 (PPFS <0.001, POS <0.001), but in Model 4, NLN exhibited no effect on PFS or OS (PPFS =0.066, POS =0.202). In Model 7, however, when putting all the clinicopathologic parameters together, only PLN was shown to be an independent prognostic factor among the six nodal staging systems for OS (P=0.049, Table 3).
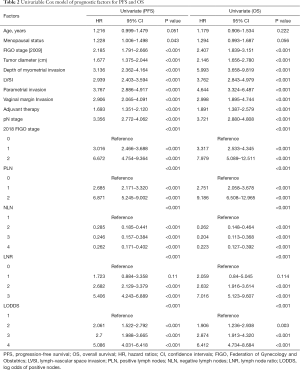
Full table
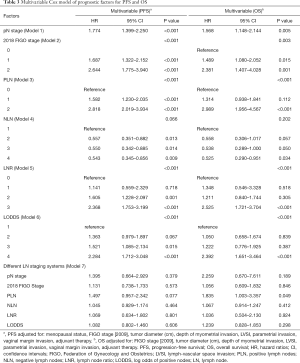
Full table
Evaluation of the prognostic significance of different LN staging systems
With regression model analysis, the LN staging system found to be the ablest in terms of prognostic discrimination then underwent assessment using iterative statistical models and comparison of C-index and AIC values. When the established categorical cut-off values were used for assessment, the prognostic performance of PLN was observed to be superior in relation to both PFS (C-index: 0.634; AIC: 33,343.83) and OS (C-index: 0.675; AIC: 34,223.11). To test whether the relative performance of PLN, NLN, LNR, and LODDS was influenced according to the chosen categorical cut-off values, we carried out repeat analysis with continuous variables in the statistical models. PLN still outperformed other nodal staging systems for both PFS (C-index: 0.638; AIC: 33,344.23) and OS (C-index: 0.679; AIC: 34,229.01), even when LN status served as a continuous variable (Table 4).

Full table
Discussion
Postoperative decision-making regarding treatment and monitoring hinge on a staging system’s ability to predict cancer patients’ long-term survival accurately. The FIGO staging criteria for cervical cancer have long been used since the early 20th century (18). However, until 2018, staging only took into account the findings of a clinical and image logical nature. For cervical cancer patients, LN status is a pivotal predictor of survival and is always used to help guide the treatment for postoperative adjuvant radiotherapy and chemotherapy (19). The newly updated 2018 FIGO staging system, saw the first-ever inclusion of LN status. However, it is still controversial that patients with only pelvic LN metastasis might have a better prognosis than those with locally advanced diseases. According to the new FIGO stage, cervical cancer patients with LN metastasis but without locally advanced tumors were all advanced diseases. Therefore, there is a tremendous necessary to explore the proper and valuable LN staging system to help guide the management of postoperative cervical cancer patients.
The importance of LN status in determining prognosis after radical surgery of cervical cancer has long been aware, and there have been a large number of different methods proposed to define an optimal LN staging system among the cervical cancer patients receiving radical surgery over the last decade. However, no consensus on the optimal LN staging method has been reached.
The TNM pN staging system is widely used in various malignancies, and the pN staging in cervical cancer only stratifies patients by the presence or absence of LN metastasis. Therefore, it can help distinguish high-risk patients at a certain level, and our results also support the fact that pN was a significant prognostic factor for both PFS and OS (Tables 1 and 3). However, pN staging is seldom used in clinical management. The LN status of the FIGO 2018 staging system is defined according to the location of metastatic LN, only including the pelvic and para-aortic LN metastasis. Yan et al. examined the new FIGO staging system’s prognostic ability and showed that the 2018 version appeared to hold value in predicting survival for patients who had risk factors after radical surgery (20). Consistent with their results, our study also revealed that the FIGO 2018 staging system turned to be a significant prognostic factor for PFS and OS. Moreover, C-index and AIC values indicated that both the pN stage and 2018 FIGO stage had predictive significance for the prognosis of cervical cancer patients, and 2018 FIGO staging system is more accurate than pN staging system (Table 4), which indicated the progressive value of 2018 FIGO staging system. However, the inadequacy of both the pN staging system and the 2018 FIGO staging system is the deficiency of the number of LN.
Previous studies compared different LN status based on the amount of LN, and of which the effect of PLN on prognosis has been demonstrated. Kwon et al. discovered PLN >3 in early-stage cervical cancer to affect disease-free survival (DFS) and distant metastasis-free survival (DMFS) (21). Similarly, Wang et al. reported that PLN ≥3 served an independent prognostic factor in OS, cancer-specific survival (CSS), and DMFS in individuals who received definitive concurrent chemoradiotherapy (CCRT) or intensity-modulated radiotherapy (IMRT) (22). Consistent with the results above, our study indicated that only PLN was shown to be an independent prognostic factor among the six nodal staging systems for OS when putting all the clinicopathologic parameters together in multivariate survival analysis (Table 3), and PLN was noted to have the best prognostic performance (Table 4).
NLN count is a surrogate indicator of surgery quality, reflecting the extent to which the LN has been dissected. Chen et al. found NLN count to have a vital influence in increasing survival for cervical cancer patients, with a 5-year survival rate of 62.8% and 80.5% when the cutoffs of the NLN count were 10 and 25, respectively (23). However, although our results showed that the NLN count had a significant influence on the 5-year PFS and OS, it was not an independent factor for PFS or OS. Moreover, both the AIC and the C-index analysis revealed that the NLN count had much less predictive power than the other five systems (Tables 3 and 4). Debate still surrounds the optimal minimum number of nodes that should be dissected because the vast proportion of women develop no nodal metastasis in the early stages of the disease, which makes the extended lymphadenectomy unnecessary (24). In our study, the proportion of LN-negative patients was 75.13%, and it is reasonable to imagine that among those patients, the intraoperative risks carried by this procedure, including vascular injuries and hemorrhaging and the postoperative formation of sequelae-like lymphocyst and lymphedema, makes it a needless procedure (25). Since our results suggested that “positive LN” was significantly more critical than “negative LN”, and a number of studies had indicated that patients did not benefit from extensive lymphadenectomy (26,27), it comes to the doubt whether it is necessary to remove these seemingly insignificant negative LN. Cervical cancer is broadly acknowledged to be improving in terms of precision, and the recent studies in sentinel LN mapping for cervical cancer also help locate the positive LN. Therefore, it brings us the thoughts in the necessity for complete lymphadenectomies when considering the relevant associated morbidity (28). Even so, large-scale and prospective studies are to be established to ensure the safety and accuracy of sentinel LN assessment to make the treatment of cervical cancer more precisely.
The main drawback of either PLN or NLN lay in fact their ability to accurately predict prognosis was predominantly influenced by the overall number of LN removed, leading to the exploration of new prognostic indicators, which can integrate all LN information into a single identifiable parameter. The LNR system covers the information of both positive LN and total LN removed and can theoretically overcome the limitations of the number-based nodal system above. Fleming et al.’s retrospective study of node-positive stage I/II cervical cancer patients who had undergone radical surgery showed an association between LNR >6.6% and worse PFS and a correlation between LNR >7.6% and worse OS (29). However, the prognostic discriminatory power of LNR seemed lacking for patients with very low or high LNR (Figure 3C). For patients with either a very low or high LNR, LODDS may be helpful in this situation, which could unlock further findings in relation to the prognoses of patients with shared LNR values but differing amounts of examined nodes. Studies had demonstrated that the LODDS system provides a superior model to the LNR system for non-small cell lung cancer, small bowel adenocarcinoma, oral squamous cell carcinoma, and gastric cancer patients (9,12,14,30). For cervical cancer, one study compared the prognostic value of the PLN, LODDS, and LNR in a relatively small cohort of cervical cancer patients who underwent radical hysterectomy and pelvic +/− para-aortic lymphadenectomy alongside adjuvant treatment (n=50). In that study, for both DFS and OS, LODDS ≥−1.05 turned out to be the only significant prognostic factor (31). However, LODDS was also had strong predictive value in our study but was not shown to be superior compared with LNR; Similar results had also been reported in pancreatic cancer (32). This result may be due to different statistical power (number of patients, clinicopathologic type), data type, and the different average number of LNs resected in these various studies.
There were several limitations to this study. First, both clinical and pathological data were obtained from a single institution, which could not bring out the diversity in the treatment of other centers. Second, our study was limited by its retrospective nature and had a relatively short mean follow-up period (39.6 months). However, unlike previous studies, our study was first to evaluate the prognostic power of six commonly used LN staging systems focusing on a large patient cohort. As far as we are aware, the included LN classification methods in the current study are the most comprehensive in cervical cancer. To provide validation of our results, further follow-up evaluation focusing on a longer period of time and large prospective studies are required.
To conclude, PLN appeared to have the most accuracy and value out of LN staging systems for CSCC patients receiving radical surgery. With the lack of specific studies comparing various LN staging methods on cervical cancer, our findings could make a valuable contribution to managing LN in cervical cancer, and we hope that more valuable data will be collected to gain a better understanding of this issue.
Acknowledgments
Funding: This work was supported by the Guidance Project of Science and Technology Commission of Shanghai Municipality (No. 17411963000).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our research received approval from the Ethics Committee at Fudan University Shanghai Cancer Center (No. 1706173-22-1707).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169-82. [Crossref] [PubMed]
- Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv72-83. [Crossref] [PubMed]
- Pfaendler KS, Chang J, Ziogas A, et al. Disparities in Adherence to National Comprehensive Cancer Network Treatment Guidelines and Survival for Stage IB-IIA Cervical Cancer in California. Obstet Gynecol 2018;131:899-908. [Crossref] [PubMed]
- Ayhan A, Aslan K, Bulut AN, et al. Is the revised 2018 FIGO staging system for cervical cancer more prognostic than the 2009 FIGO staging system for women previously staged as IB disease? Eur J Obstet Gynecol Reprod Biol 2019;240:209-14. [Crossref] [PubMed]
- Tokunaga H, Shimada M, Ishikawa M, et al. TNM classification of gynaecological malignant tumours, eighth edition: changes between the seventh and eighth editions. Jpn J Clin Oncol 2019;49:311-20.
- Matsuo K, Machida H, Mandelbaum RS, et al. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol 2019;152:87-93. [Crossref] [PubMed]
- Morales-Oyarvide V, Rubinson DA, Dunne RF, et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer 2017;117:1874-82. [Crossref] [PubMed]
- Polterauer S, Schwameis R, Grimm C, et al. Prognostic value of lymph node ratio and number of positive inguinal nodes in patients with vulvar cancer. Gynecol Oncol 2017;147:92-7. [Crossref] [PubMed]
- Safi AF, Kauke M, Grandoch A, et al. The importance of log odds of positive lymph nodes for locoregional recurrence in oral squamous cell carcinoma. Oral Oncol 2017;72:48-55. [Crossref] [PubMed]
- Tarantino I, Warschkow R, Hackert T, et al. Staging of pancreatic cancer based on the number of positive lymph nodes. Br J Surg 2017;104:608-18. [Crossref] [PubMed]
- Wu H, Liu C, Xu M, et al. Prognostic value of the number of negative lymph nodes in esophageal carcinoma without lymphatic metastasis. Thorac Cancer 2018;9:1129-35. [Crossref] [PubMed]
- Zhou YY, Du XJ, Zhang CH, et al. Comparison of three lymph node staging schemes for predicting the outcome in patients with small bowel adenocarcinoma: A population-based cohort and international multicentre cohort study. EBioMedicine 2019;41:276-85. [Crossref] [PubMed]
- Wang J, Li J, Chen R, et al. Contribution of lymph node staging method and prognostic factors in malignant ovarian sex cord-stromal tumors: A world wide database analysis. Eur J Surg Oncol 2018;44:1054-61. [Crossref] [PubMed]
- Wang ZX, Qiu MZ, Jiang YM, et al. Comparison of prognostic nomograms based on different nodal staging systems in patients with resected gastric cancer. J Cancer 2017;8:950-8. [Crossref] [PubMed]
- Pei JP, Zhang CD, Fan YC, et al. Comparison of Different Lymph Node Staging Systems in Patients With Resectable Colorectal Cancer. Front Oncol 2019;8:671. [Crossref] [PubMed]
- Small W Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer 2017;123:2404-12. [Crossref] [PubMed]
- Han X, Wen H, Ju X, et al. Predictive factors of para-aortic lymph nodes metastasis in cervical cancer patients: a retrospective analysis based on 723 para-aortic lymphadenectomy cases. Oncotarget 2017;8:51840-7. [PubMed]
- Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129-35. [Crossref] [PubMed]
- Wipperman J, Neil T, Williams T. Cervical Cancer: Evaluation and Management. Am Fam Physician 2018;97:449-54. [PubMed]
- Yan DD, Tang Q, Chen JH, et al. Prognostic value of the 2018 FIGO staging system for cervical cancer patients with surgical risk factors. Cancer Manag Res 2019;11:5473-80. [Crossref] [PubMed]
- Kwon J, Eom KY, Kim YS, et al. The Prognostic Impact of the Number of Metastatic Lymph Nodes and a New Prognostic Scoring System for Recurrence in Early-Stage Cervical Cancer with High Risk Factors: A Multicenter Cohort Study (KROG 15-04). Cancer Res Treat 2018;50:964-74. [Crossref] [PubMed]
- Wang SC, Lin LC, Kuo YT, et al. Radiographic Number of Positive Pelvic Lymph Nodes as a Prognostic Factor in Cervical Cancer Treated With Definitive Concurrent Chemoradiotherapy or Intensity-Modulated Radiotherapy. Front Oncol 2018;8:546. [Crossref] [PubMed]
- Chen Y, Zhang L, Tian J, et al. Combining the negative lymph nodes count with the ratio of positive and removed lymph nodes can better predict the postoperative survival in cervical cancer patients. Cancer Cell Int 2013;13:6. [Crossref] [PubMed]
- Höckel M, Horn LC, Tetsch E, et al. Pattern analysis of regional spread and therapeutic lymph node dissection in cervical cancer based on ontogenetic anatomy. Gynecol Oncol 2012;125:168-74. [Crossref] [PubMed]
- Togami S, Kawamura T, Fukuda M, et al. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Jpn J Clin Oncol 2018;48:1036-40. [Crossref] [PubMed]
- Suprasert P, Charoenkwan K, Khunamornpong S. Pelvic node removal and disease-free survival in cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet 2012;116:43-6. [Crossref] [PubMed]
- Ditto A, Martinelli F, Lo Vullo S, et al. The role of lymphadenectomy in cervical cancer patients: the significance of the number and the status of lymph nodes removed in 526 cases treated in a single institution. Ann Surg Oncol 2013;20:3948-54. [Crossref] [PubMed]
- Salvo G, Ramirez PT, Levenback CF, et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol Oncol 2017;145:96-101. [Crossref] [PubMed]
- Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol 2015;136:48-53. [Crossref] [PubMed]
- Deng W, Xu T, Wang Y, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer 2018;122:60-6. [Crossref] [PubMed]
- Kwon J, Eom KY, Kim IA, et al. Prognostic Value of Log Odds of Positive Lymph Nodes after Radical Surgery Followed by Adjuvant Treatment in High-Risk Cervical Cancer. Cancer Res Treat 2016;48:632-40. [Crossref] [PubMed]
- Riediger H, Kulemann B, Wittel U, et al. Prognostic Role of Log Odds of Lymph Nodes After Resection of Pancreatic Head Cancer. J Gastrointest Surg 2016;20:1707-15. [Crossref] [PubMed]


