Evaluating the effectiveness of targeted therapies for thyroid carcinoma: an updated meta-analysis
Introduction
Thyroid carcinoma is the most common malignant carcinoma of the endocrine system. In the past few years, the incidence of thyroid carcinoma has increased at a greater rate than other tumors, and the number of confirmed cases of thyroid carcinoma is more than double of that in the previous year (1). Surgery is the preferred clinical treatment for thyroid carcinoma, but a few other treatments types are also relatively successful. For most patients with early thyroid carcinoma, the prognosis is good after surgery, postoperative thyroid stimulating hormone (TSH) inhibition therapy, and radioactive iodine (RAI) therapy. Due to the success of these therapies, the 20-year survival rate is over 90%. However, there still remain a number of refractory thyroid carcinomas, including advanced, recurrent, metastatic thyroid, and anaplastic thyroid carcinomas. As a consequence of the lack of treatment for these conditions, the survival of these patients is very unsatisfactory (2). Targeted drugs that can compensate for the failure of traditional treatment have given hope for the treatment of these thyroid carcinoma types, and finding effective targeted treatments is critical to improving its prognosis.
The current molecular targets for the treatment of thyroid carcinoma include: a variety of kinases, BRAF, AKT, mTOR, VEGFR, and MEK (3-5). Compared with traditional chemotherapy drugs, these drugs are characterized by high efficiency and low toxicity. Early small-scale clinical trials have shown that the use of targeted drugs, such as the multi-target tyrosine kinase inhibitors like axitinib, imatinib, sorafenib, etc. can control the progression of thyroid carcinoma to some extent. Therefore, targeted drug therapy is considered to be one of the most promising treatments for thyroid carcinoma (6,7). At present, most of the studies on molecular targeted therapy for advanced thyroid carcinoma have been single-arm experiments, and there only a few prospective randomized controlled trials have been conducted. This study therefore aimed to use a meta-analysis to evaluate the efficacy and safety of molecular targeted therapy in thyroid carcinoma with the hope of providing a theoretical basis for its application.
Methods
Search strategy
Two authors independently searched all the English-language literature in the Medline, PubMed, EMBASE, ClinicalTrails.gov, Cochrane Library, and Ovid electronic databases published as of September 1, 2019 by. For our search strategy, the following keywords were used in all fields: “thyroid carcinoma” or “thyroid carcinoma” or “thyroid neoplasm” or “thyroid tumor” and “targeted therapy”. The electronic search was supplemented by a manual search of academic papers, dissertations, and conference abstracts published by various magazines.
Study selection
The inclusion criteria of eligible studies were as follows: (I) the literature research methods was a randomized controlled study (RCT), and the efficacy and safety of targeted therapy in thyroid carcinoma was analyzed; (II) studies included more than 50 patients; (III) all the included documents were available for data extraction; (IV) there were cases and controls in each study, and the controls were all placebo. Studies were excluded according to the following criteria: (I) to avoid the extraction of duplicate data, only the latest or most complete report was included if the same case population was published in more than one journal; (II) studies were not English language; (III) the full text of studies could not be accessed on-line or with request to the authors.
Quality assessment and data extraction
The utility index was the patient’s progression-free survival (PFS) rate. The safety index was the adverse reaction of the drug, which mainly included the incidence of various adverse reactions with a severity of ≥3 and the total incidence of all adverse reactions. The quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS) (8), while the Jadad scoring system was used for RCTs (9).
Statistical analysis
All the statistical analyses in this review were conducted using RevMan 5.3 software. Mean data were measured as mean difference (MD), and count data were expressed as odds ratio (OR or RR) with a 95% confidence interval (95% CI). If the test results did not show statistical heterogeneity (I2 ≤50%), the data were combined and analyzed using a fixed effects model. If there was statistical heterogeneity between the two study groups without clinical heterogeneity, a random effects model was used for analysis. A sensitivity analysis of the literature was performed if heterogeneity arose from low-quality studies, while a declarative analysis was used if the heterogeneity between the two groups was too large or the source of the data could not be found.
Results
Eligible articles
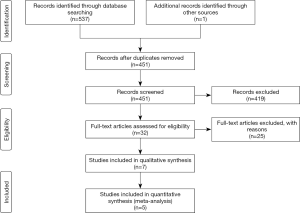
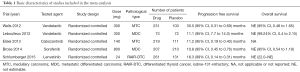
According to the search strategy, a total of 537 related articles were retrieved. After reading the title and abstract of the literature, the screening criteria were used for screening, resulting in 7 RCT research literatures which were read and analyzed. After 2 duplicated data sets were excluded, 5 RCT studies remained (Figure 1) comprising a total of 991 cases in drug group and 624 cases in the placebo group (Table 1).
Full table
Study characteristics and quality
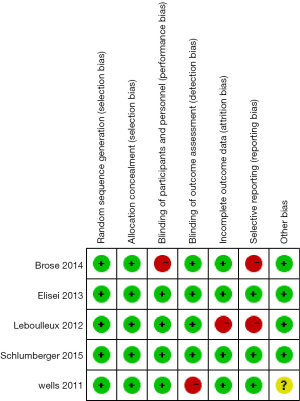
Quality analysis was performed on the 5 RCT studies included using the Cochrane Risk Bias Assessment Tool. All 5 articles were RCTs (10-14). Four of the studies were rated as high quality and one was evaluated as medium quality (Figure 2).
Results of statistical meta-analysis
Drug efficacy analysis
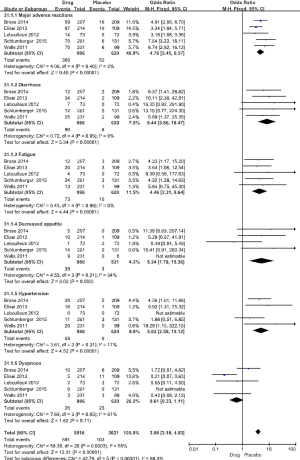
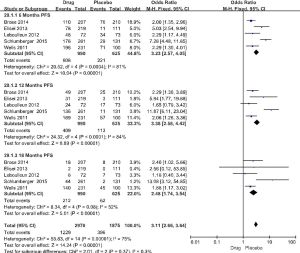
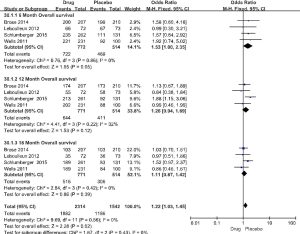
The 5 RCT studies included 991 cases in the drug group and 624 cases in the placebo group. The primary evaluation index of efficacy was PFS, and the secondary indicator was overall survival (OS). In the 5 studies, the PFS for the drug group was 10.8–30.5 months, compared with only 4.0–19.3 months for the placebo group (Table 1). In the respective comparison of PFS at 6, 12, and 18 months between the drug and placebo group, the PFS rate of the drug group was significantly improved (6 months PFS: OR =3.23, 95% CI: 2.57 to 4.05, P<0.00001, 12 months PFS: OR =3.38, 95% CI: 2.58 to 4.42, P<0.00001, 18 months PFS: OR =2.48, 95% CI: 1.74 to 3.54, P<0.00001) (Figure 3). OS did not differ significantly in the study (6 months: OR =1.53, 95% CI: 1.00 to 2.35, P=0.05, 12 months: OR =1.26, 95% CI: 0.94 to 1.69, P=0.12, 18 months: OR =1.11, 95% CI: 0.87 to 1.42, P=0.39) (Figure 4).
Security analysis
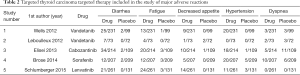
The major adverse reactions in 5 studies are shown in Table 2. The most frequent treatment-emergent adverse events in the sorafenib group were hand-foot skin reaction (76.3%), diarrhea (6.6%), alopecia (67.1%), and rash or desquamation (50.2%) (10). Common cabozantinib-associated adverse events included diarrhea, palmar-plantar erythrodysesthesia, decreased weight and appetite, nausea, and fatigue, which resulted in dose reductions in 79% of patients and holds in 65% of patients (14). The most common grade 3 or worse adverse events were QTc prolongation [10 (14%) of 73 patients in the vandetanib group vs. none in the placebo group], diarrhea [7 (10%) vs. none], asthenia [5 (7%) vs. 3 (4%)], and fatigue [4 (5%) vs. none] (13). Treatment-related adverse effects of any grade, which occurred in more than 40% of patients in the lenvatinib group, were hypertension (in 67.8% of the patients), diarrhea (in 59.4%), fatigue or asthenia (in 59.0%), decreased appetite (in 50.2%), decreased weight (in 46.4%), and nausea (in 41.0%) (12). Common adverse events (any grade) occurred more frequently with vandetanib compared with placebo, including diarrhea (56% vs. 26%), rash (45% vs. 11%), nausea (33% vs. 16%), hypertension (32% vs. 5%), and headache (26% vs. 9%) (11). However, in the study, we mainly analyzed the incidence of serious ≥3 adverse reactions.
Full table
The incidence of adverse reactions in the drug group was significantly higher than that in the placebo group (OR =10.06, 95% CI: 7.46 to 13.55, P<0.00001) (Figure 5), and the subgroup of adverse reactions was significantly higher than that in the placebo group.
Diarrhea
There was no statistical heterogeneity between the 5 studies (P=0.95, I2 =0%). Meta analysis showed that the incidence of diarrhea in the drug group was significantly higher than that of the control group (OR =8.44, 95% CI: 3.86 to 18.47, P<0.000001) (Figure 5).
Fatigue
There was no statistical heterogeneity between the 5 studies (P=0.98, I2 =0%). Meta analysis showed that the incidence of fatigue in the drug group was significantly higher than that of the control group (OR =4.46, 95% CI: 2.31 to 8.64, P<0.00001) (Figure 5).
Decreased appetite
There was no statistical heterogeneity between the 5 studies (P=0.21, I2 =34%). Meta analysis showed that the incidence of decreased appetite in the drug group was significantly higher than that of the control group (OR =5.24, 95% CI: 1.79 to 15.36, P=0.003) (Figure 5).
Hypertension
There was no statistical heterogeneity between the 5 studies (P=0.31, I2 =17%). Meta analysis showed that the incidence of hypertension in the drug group was significantly higher than that of the control group (OR =5.03, 95% CI: 2.50 to 10.12, P<0.00001) (Figure 5).
Dyspnea
There was statistical heterogeneity between the 5 studies (P=0.05, I2 =61%). Meta analysis showed that the incidence of dyspnea in the drug group was not significantly different than that of the control group (OR =0.61, 95% CI: 0.33 to 1.11, P=0.11) (Figure 5).
Discussion
The objective of the current study was to evaluate the effectiveness of targeted therapies for thyroid carcinoma. Five RCTs were included. The meta-analysis indicated that targeted therapies, as compared with placebo, were associated with more significant improvements in PFS and in the response rate among patients with thyroid carcinoma. Patients who received targeted therapies had more adverse effects, but the patients were still tolerant. To our knowledge this is the first systematic review and meta-analysis that evaluates the effectiveness of targeted therapies for thyroid carcinoma.
Thyroid carcinoma is the most common malignant tumor of the endocrine system, and has shown the fastest growing incidence in recent years, with age standardized (world population) rates of 6.10 and 1.90 per 100,000 persons (15). Thyrotropin (TSH) inhibition or RAI treatment after surgery, can have the most benefit as a routine treatment for thyroid carcinoma, with the 5-year survival rate of patients with thyroid carcinoma increasing to 97.8% (16). However, 7–23% of patients develop distant metastases (17), and so there are still a large number of refractory thyroid carcinomas, such as advanced, recurrent, metastatic thyroid, medullary, and anaplastic thyroid carcinoma. Due to a lack of treatment, the survival of patients is very unsatisfactory (18,19). In the context of today’s precision medicine era, molecular targeted therapy is increasingly valued and is playing an ever-growing role in the treatment of thyroid carcinoma; its development has offered these patients a new hope.
At present, most of the research on targeted therapy of thyroid carcinoma is Phase I study and Phase II single-arm study. There are still a few Phase III and RCT clinical studies. Therefore, this study evaluated the efficacy and safety of targeted drugs by meta-analysis. The drugs included in the study were lenvatinib, vandetanib, cabozantinib, and sorafenib (10-14). Sorafenib, a tyrosine kinase inhibitor that inhibits VEGFRs 1, 2, and 3, PDGFR β, Raf-1, RET, and BRAF, was approved by the U.S. Food and Drug Administration (FDA) for the treatment of iodine-131-refractory thyroid carcinoma on the basis of results of a phase 3 trial showing a 5-month improvement in median PFS (10). Lenvatinib is an oral, multi-targeted tyrosine kinase inhibitor of the VEGFRs 1, 2, and 3; the FGFRs 1 through 4; and the PDGFR α, RET, and KIT signaling networks (20,21). Cabozantinib is a TKI that targets three relevant pathways in MTC: MET, VEGFR2, and RET (22). Vandetanib is a once-daily oral agent that selectively targets RET, VEGFR, and EGFR signaling (23,24).
This study showed that targeted drugs effectively improve patients’ PFS (6 months PFS: OR =3.23, 95% CI: 2.57 to 4.05, P<0.00001, 12 months PFS: OR =3.38, 95% CI: 2.58 to 4.42, P<0.00001, 18 months PFS: OR =2.48, 95% CI: 1.74 to 3.54, P<0.00001). The total PFS of drug group was more than 3 times that of the control group(OR =3.11, 95% CI: 2.66 to 3.64, P<0.00001) (Figure 3), indicating that the targeted therapy has a stable therapeutic effect.
This study also discussed several serious adverse events (incidence of serious adverse event ≥ grade 3) with the highest incidence of each drug group, the incidence of adverse reactions in the drug group was significantly higher than that in the placebo group (OR =10.06, 95% CI: 7.46 to 13.55, P<0.00001) (Figure 5). Serious adverse reactions common to the four drugs included diarrhea, fatigue and high blood pressure. Hypertension was the most common adverse event in the lenvatinib group (in 67.8% of the patients), and the most common adverse events (any grade) that occurred with vandetanib were diarrhea [seven (10%) vs. none]. The most frequent treatment-emergent adverse events in the sorafenib group were hand-foot skin reaction, but diarrhea was the most adverse event in cabozantinib, which is consistent with the previous research (25-28). By supporting treatment or reducing the dose of the drug, almost all patients can tolerate these adverse reactions until the end of the trial. The adverse reactions were significantly reduced after the reduction of the drug dose (11,12,14), but there was no RCT to prove the efficacy at low doses, and further research is needed.
There are still many targeted drugs under study. In 2011, the first BRAFV600E targeted inhibitor, vemurafenib, was approved by the US FDA. A current phase II clinical trial on Willofini is also underway (29).
The MEK1/2 inhibitor AZD6244 (selumetinib) is a potent and highly selective MEK1 inhibitor that is considered to be an adjuvant therapy for patients with inadequate response to RAI. In 2013, selumetinib was awarded the orphan drug qualification by the US FDA for the treatment of advanced differentiated thyroid carcinoma (DTC), demonstrating potential in the treatment of radioiodine-refractory (RR)-DTC patients (30). In addition, there are many other targets for the treatment of refractory thyroid carcinoma, such as RET, ALK, RAS, MEK, BRAF, MEK1/2, histone deacetylase (HDAC) and mechanistic target of rapamycin (MTOR). Phase I and phase II trials of these targeted drugs are ongoing.
There are several limitations in our meta-analysis. First, although all the included studies were prospective RCTs, the patient population and study sample were small, and thus bias was inevitable. Secondly, there was no stratified analysis of factors that might have influenced effectiveness, such as gender, age, and type of genetic mutation. Finally, the confounding effect of different experience of physician in different medical institutions was not accounted for.
In summary, the findings of this study indicate that targeted drugs can significantly prolong PFS in patients with thyroid carcinoma. Although the incidence of adverse reactions was significantly higher than that of the control group, the patient was still tolerant. At present, most of the targeted research on refractory thyroid carcinoma is in the clinical trial stage, and there is still no strong evidence to confirm its clinical effect. Therefore, the potential risks and benefits must be considered comprehensively before targeted therapy can proceed. It is believed that with the emergence of more targeted therapies, breakthroughs can be made in the clinical treatment of refractory thyroid carcinoma with more substantial benefits being brought to patients.
Acknowledgments
Funding: City School Science and Technology Research Project of Nanchong (Grant number: 18SXHZ0323).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Carling T, Udelsman R. Thyroid carcinoma. Annu Rev Med 2014;65:125-37. [Crossref] [PubMed]
- Spitzweg C, Bible KC, Hofbauer LC, et al. Advanced radioiodine-refractory differentiated thyroid carcinoma: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014;2:830-42. [Crossref] [PubMed]
- Smith NR, Baker D, James NH, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin cancer Res 2010;16:3548-61. [Crossref] [PubMed]
- Wells SA Jr, Santoro M. Targeting the RET Pathway in Thyroid cancer. Clin Cancer Res 2009;15:7119-23. [Crossref] [PubMed]
- Chai L, Han D, Li J, Lv Z. The construction and analysis of gene co-expression network of differentially expressed genes identifies potential biomarkers in thyroid cancer. Transl Cancer Res 2018;7:1235-43. [Crossref]
- Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid carcinoma: molecular pathogenesis and emerging therapies. Endocr Relat Cancer 2009;16:17-44. [Crossref] [PubMed]
- Kojic SL, Strugnell SS, Wiseman SM. Anaplastic thyroid carcinoma: a comprehensive review of novel therapy. Expert Rev Anticancer Ther 2011;11:387-402. [Crossref] [PubMed]
- Lee J, Kim HS, Shin SH, et al. Efficacy and safety of fluconazole prophylaxis in extremely low birth weight infants: multicenter pre-post cohort study. BMC Pediatr 2016;16:67. [Crossref] [PubMed]
- McCormick F, Cvetanovich GL, Kim JM, et al. An assessment of the quality of rotator cuff randomized controlled trials: utilizing the Ja dad score and CONSORT criteria. J Shoulder Elbow Surg 2013;22:1180-5. [Crossref] [PubMed]
- Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid carcinoma: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319-28. [Crossref] [PubMed]
- Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid carcinoma. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid carcinoma: a randomised, double-blind, phase 2 trial. Lancet Oncol 2012;13:897-905. [Crossref] [PubMed]
- Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid carcinoma. J Clin Oncol 2013;31:3639-46. [Crossref] [PubMed]
- Siraj AK, Beg S, Jehan Z, et al. The role of HER2 overexpression in Middle Eastern papillary thyroid cancer. Transl Cancer Res 2017;6:366-73. [Crossref]
- Sun H, Carcoforo P, Dionigi G. Puzzle over active surveillance for micropapillary thyroid carcinoma. Ann Transl Med 2018;6:132. [Crossref] [PubMed]
- Shoup M, Stojadinovic A, Nissan A, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg 2003;197:191-7. [Crossref] [PubMed]
- Spitzweg C, Bible KC, Hofbauer LC, et al. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014;2:830-42. [Crossref] [PubMed]
- Shaha AR. Recurrent differentiated thyroid carcinoma. Endocr Pract 2012;18:600-3. [Crossref] [PubMed]
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664-71. [Crossref] [PubMed]
- Matsui J, Funahashi Y, Uenaka T, et al. Multikinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 2008;14:5459-65. [Crossref] [PubMed]
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298-308. [Crossref] [PubMed]
- Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 2002;62:7284-90. [PubMed]
- Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 2002;62:4645-55. [PubMed]
- de Castroneves LA, Negrão MV, de Freitas RM, et al. Sorafenib for the treatment of progressive metastatic medullary thyroid carcinoma: efficacy and safety analysis. Thyroid 2016;26:414-9. [Crossref] [PubMed]
- Robinson BG, Paz-Ares L, Krebs A, et al. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid carcinoma. J Clin Endocrinol Metab 2010;95:2664-71. [Crossref] [PubMed]
- Hewett Y, Ghimire S, Farooqi B, et al. Lenvatinib-A multikinase inhibitor for radioiodine- refractory differentiated thyroid carcinoma. J Oncol Pharm Pract 2018;24:28-32. [Crossref] [PubMed]
- Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid carcinoma. J Clin Oncol 2011;29:2660-6. [Crossref] [PubMed]
- Cabanillas ME, Patel A, Danysh BP, et al. BRAF inhibitors: experience in thyroid carcinoma and general review of toxicity. Horm Cancer 2015;6:21-36. [Crossref] [PubMed]
- Wilson C. Selumetinib promotes radioiodine uptake in thyroid carcinomas. Nat Rev Endocrinol 2013;9:253.30. Wilson C. Selumetinib promotes radioiodine uptake in thyroid carcinomas. Nat Rev Endocrinol 2013;9:253. [Crossref] [PubMed]