
Effect of combined low-dose oral prednisone with beta-adrenergic receptor antagonists for refractory infantile hemangiomas: retrospective cohort study in 76 patients
Introduction
Infantile hemangiomas (IHs) are the most common tumor of infancy, with a prevalence estimated at 1–10% of the infants worldwide. IHs most often appear on the head, neck, and trunk and some patients will experience complications, such as pain, ulceration and functional limitation (1). Previous studies indicate that decreasing gestational age, low birth weight, and female predominance are closely associated with higher IH incidence (2). In 2008, Léauté-Labrèze et al. reported the success to treat hemangioma with propranolol (3), which leads to a revolution of IH management, and the use of propranolol has come to the forefront because of its efficacy and minimal side effect. Since then, propranolol and other beta-adrenergic receptor antagonists have become the first-line treatment instead of corticosteroid therapy. For superficial IHs, topical beta-adrenergic receptor antagonists have been commonly used (4-6) and oral propranolol has become the standard treatment for high-risk and deep IH (7). However, the clinical response rate of the first-line treatment with propranolol is around 90% and there are still cases with large size or serious complications or low sensitivity to propranolol can not receive notable improvement after beta-adrenergic receptor antagonists (7,8). Therefore, a combination of prednisone and beta-adrenergic receptor antagonists is brought to the forefront (9-13) by several investigators. There were few studies in this filed, however, higher dose or longer duration of prednisone were used, or fewer cases were reported.
In this study, we summarized our experiences of treating refractory IH patients with a combination of low dose and short duration prednisone and beta-adrenergic receptor antagonists, which demonstrated a promising perspective for these cases.
Methods
Study design
We conducted the retrospective cohort study of refractory IH patients between June 2014 and June 2018 at our outpatient clinic at the Department of Oral and Maxillofacial Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Patients that fulfilled our inclusion and exclusion criteria were consecutively enrolled. The inclusion criteria was: patients who received oral propranolol at least 1 month after treatment initiation; the hemangioma score value was evaluated before and after oral propranolol treatment, and the hemangioma score variations were defined as ≤1; parents or guardian of patients gave written informed consent. The exclusion criteria included hypersensitivity to propranolol, heart defects or arrhythmia. Patients were followed up through outpatients and by mobile application or phone call, if necessary.
This study was carried out in accordance with the recommendations of Declaration of Helsinki. The protocol was approved by the Institute Review Board of Shanghai Ninth People’s Hospital (SH9H-2019-T272-1). All parents or guardian of participants of this study gave written informed consent in accordance with the Declaration of Helsinki for the participation in the study, and the publication of identifiable images at the initiate prescription.
Treatment regimen
All patients were given oral propranolol (Jiangsu Yabang Aipusen Pharmaceutical Industry Limited Company, China) at a dose of 2.0 mg/kg/day. For patients with superficial or compound IHs, topical 0.5% timolol maleate eye drops (Bausch Lomb Pharmaceutical Industry Limited Company, China) was used three times a day at an interval of 8 hours.
Patients were divided into two groups retrospectively depending on whether received additional oral prednisone. For patients in group 1, prednisone (Shanghai Sine Pharmaceutical Laboratories Company, China) was used only for one month at a dose of 1 mg/kg every other day and other drug administrations were as the same as the patients (group 2) who received monotherapy of beta-adrenergic receptor antagonists. The drug administrations were continued until objective goals were obtained or no further improvement was achieved.
All patients underwent a thorough physical examination prior to the treatment, including cardiovascular examination, ultrasound investigation, and clinical photography. During the treatment, cardiovascular examination (including heart rate and blood pressure) were carried out before and after the initiate treatment. Then cardiovascular examination was repeated every one month as a routine. Weight and height were also recorded before and after one month’s prednisone in patients of group 1.
Outcome measurement
Until now, no standardized or validated method exists for outcome measurement of hemangiomas. Because the goal of treatment was minimizing functional and cosmetic impairment, we modified the semi-quantitative hemangioma score system (6) to evaluate the therapeutic effects. The outcomes of each patient were evaluated based on the improvement of color, size, surface consistency, firmness, depth and functional disturbance (as shown in Table 1). Evaluation of the improvement was conducted twice by the other two independent physicians when the first visit, the diagnosis of refractory IH made, one month after oral prednisone and after the full course of treatment. Therapeutic response was assessed by the percentage of changes in score values as follows: excellent response (75–100%), good response (50–75%), fair response (25–50%) and poor response (0–25%).

Full table
Statistical analysis
Statistical analysis was performed using the SPSS software package (version 16.0; SPSS, Chicago, IL) and R program. Descriptive data were expressed as number, percentage, or means ± standard deviation. Wilcoxon test was used to compare the clinical response at different groups and based on the percentage of changes in score values. Spearman rank correlation was used to analyze the correlation between clinical characteristics and the percentage of changes in score values. Orient logistic regression analysis was used to determine the important factors to the treatment response. P value <0.01 were considered significant.
Results
Clinical and histological features
Eighty-six patients were identified as eligible for the study because their hemangioma score variations were defined as ≤1. Ten were excluded because of missing data. The remaining 76 made up the cohort. The mean age when the diagnosis of refractory IH was mad was 5.52 (±2.85) months, and 54 patients (71.05%) were female. The detailed histological features were in Table 2.
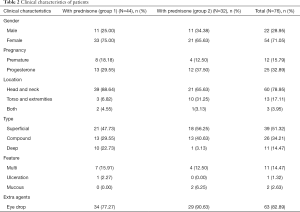
Full table
Based on the treatment regimen, the cohort was divided into 2 groups, forty-four patients who received combined treatment of propranolol and prednisone were assigned to group 1, and thirty-two patients who received monotherapy of propranolol were assigned to group 2 as control. Patients with superficial, compound but no mucosa IHs (n=63) received extra topical 0.5% timolol maleate eye drops, among them, thirty-four were in group 1 and twenty-nine were in group 2.
Therapeutic outcomes
The therapeutic outcomes were accessed by the average score variation percentage, which was the proportion of the score variation in the initial hemangioma score.
- The average score variation percentage after combined treatment in group 1 was 66.41% (23.03%), and 44.97% (17.57%) after monotherapy in group 2. The significant difference was observed between the two groups (P=0.000).
- In group 1, 35 (79.55%) patients showed immediate improvement after one month’s prednisone, and the mean score variation percentage was 26.12% (±11.53%).
- The average entire treatment duration was 9 (±2.06) months in group 1 and 8.81 (±5.54) months in group 2. No significant difference was observed between the two groups (P=0.320).
- For patients with superficial, compound, multiple IHs who received extra topical 0.5% timolol maleate eye drops (n=63), there was also significant difference between average score variation percentage in group 1 (n=34, 62.81%±22.47%) and those in group 2 (n=29, 44.38%±17.73%), with 0.002 as the P value.
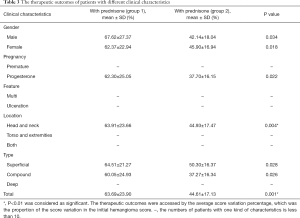
Full table
Correlation analysis
We performed the correlation analysis between the score variation percentage and the entire treatment duration, the age at initiate treatment in both groups; between the score variation percentage and the age at initiate prednisone, the duration of treatment before and after prednisone in group 1 (Table 4).
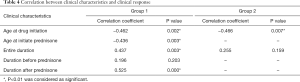
Full table
- The age at initiate prednisone showed significant correlation with score variation percentage (r=−0.436, P=0.003) in group 1.
- The age at initiate treatment showed significant correlation with score variation percentage both in group 1 (r=−0.462, P=0.002) and group 2 (r=−0.466, P=0.007).
- The entire treatment duration showed significant correlation with score variation percentage in group 1 (r=0.437, P=0.003) but not in group 2 (r=0.173, P=0.345).
- In group 1 the duration of treatment after prednisone was significantly correlated with the improvement (r=0.525, P=0.000) but that before prednisone showed no correlation with the score variation percentage (r=0.196, P=0.203).
Addition of prednisone and the age at drug initiation were the most important factors
We performed ordinal logistic regression analysis on the overall outcomes in all patients with all clinical characteristics and different treatment regimens (gender, location, premature or not, with or without progesterone, type, feature, the age at drug initiation, the entire duration of propranolol, addition of prednisone). The results showed that addition of prednisone (coefficient: −1.795, P=0.005) and the age at drug initiation (coefficient: −1.592, P=0.001) were the most important factors to the overall outcomes (Table 5).
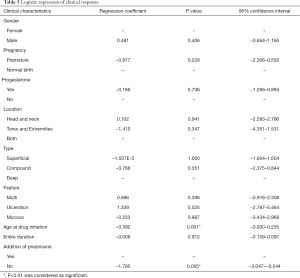
Full table
Complications
No systemic adverse effects were noted during treatment. No significant increase in weight or height was recorded after one month’s low dose prednisone in group 1. Local side effects were observed in 3 patients who used topical timolol, two patients experienced pruritus and 1 patient developed excoriations. These minor side effects resolved within 10 days without specific treatment.
Follow-up
The follow-up period after treatment ranged from 10 months to 2.5 years. No patient experienced recurrence or rebound growth of the lesion at the last follow-up. Typical images with excellent response were shown in Figures 1-4.
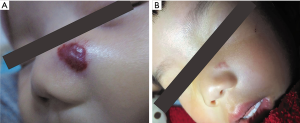
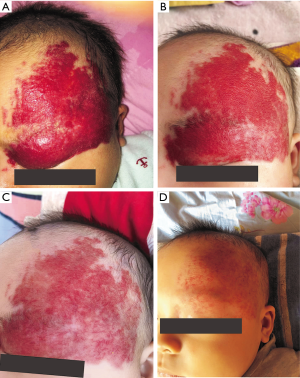
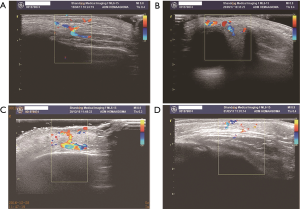
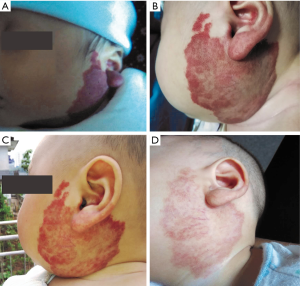
Discussion
Prednisone used to be the first-line therapy for IHs until numerous studies support the efficacy and reduced side effects of propranolol. Since 2008 beta-adrenergic receptor antagonists have become the first choice instead of corticosteroid therapy. However, monotherapy of propranolol can not always achieve notable improvement for the refractory IHs with large size or serious complications or low sensitivity to propranolol, as reported by other investigators (7,8).
In this study, we retrospectively analyzed the refractory IH treatment cohort, divided the cohort into 2 groups depending on the treatment regimens and compared the therapeutic outcomes between the combined additional prednisone group and monotherapy group. As shown in the result section, patients in combined treatment received significant improvement than those in the monotherapy group. Besides, 79.55% of patients showed immediate improvement after one month’s prednisone. Patients with superficial, compound IHs were given extra topical 0.5% timolol maleate eye drops as the regular treatment. Our results showed that for those patients, treatment of oral propranolol and topical 0.5% timolol maleate eye drops with additional prednisone also achieved better outcomes than those without prednisone. This finding is in agreement with our hypothesis and consistent with other studies (9,11,13).
Correlation analysis showed that the age to initiate prednisone was significantly negatively correlated with the improvement in the combination treatment group, which suggested that additional prednisone should be given in the early stage when no notable improvement was achieved after the beta-adrenergic receptor antagonists treatment. Meanwhile, the age at initiate treatment showed significant correlation with score variation percentage both in group 1 and group 2, which indicated that refractory IHs should be treated as early as possible even though the combination therapy was not chosen.
The entire treatment duration showed a significant correlation with the score variation percentage in group 1 but not in group 2. In group 1 the duration of treatment after prednisone was significantly correlated with the improvement but that before prednisone showed no correlation with outcomes. Considering the natural involution process, the treatment of our patients usually continued after the patient was one year’s old. The earlier the patient began the treatment, the longer the entire duration and duration after the prednisone would be. Besides, since there was no significant difference on the treatment duration observed between the two groups, we believe that the additional prednisone would not shorten the entire treatment duration and administration of beta-adrenergic receptor antagonists until one year old would be necessary for all the patients.
Considering the confounding factors, we performed ordinal logistic regression analysis on the overall outcomes in all patients with all clinical characteristics and different treatment regimens. The results also suggested that addition of prednisone and early treatment were the most two important factors to the overall outcomes.
As a non-selective β blocker, the effect of propranolol on IHs have been attributed to vasoconstriction, angiogenesis inhibition, and apoptosis induction on many types of cell, especially hemangioma endothelial cells (HemECs) (14,15). It is reported that the sensitivity to β blockers differed from races and the quantity of propranolol bound to plasma protein resulted in different pharmacological activities (16). The different subtypes of β-adrenoceptor (β-AR) also contributed to the different sensitivity of propranolol (17-19). These may explain some of the fair or poor response to propranolol and the better outcomes for patients receiving a combination of propranolol and prednisone in our study. As the previous first-line therapy, prednisone was reported to be effective for IHs based on the ability to suppress angiogenesis and macrophages and downregulate vascular endothelial growth factor and basic fibroblast growth factor (20). When the current first-line medicine, beta-adrenergic receptor antagonists, cannot receive notable improvement, the addition of prednisone may have a synergistic inhibitory effect for refractory IHs.
In recent years, there were few case reports suggesting the efficiency of the combination treatment with oral corticosteroids and propranolol in the complicated, ulcerated IH (9,10). To the best of our knowledge, this is most cases work that reports combined medication of oral prednisone with propranolol for refractory infantile hemangiomas. Some studies also revealed the promising effects of the additional oral prednisone; however, higher dose or longer duration were used, or fewer cases were reported. Nieuwenhuis et al. reported 21 cases in whom propranolol failed or was contraindicated but oral prednisone received good responses in 62% patients, with a dose of 3 mg/kg/day (13). In 2015, Aly et al. suggested that combining propranolol with corticosteroids gives a faster response and should be considered in treating life- or function-threatening hemangiomas, with a dose of 2 mg/kg/day (11). Oral prednisolone with a low dose of 1 mg/kg/day was given to the IH patients and received better outcomes in Anjum’s research, while the duration was 3 months (12).
Compared to the previous studies, our research explored the effective theory in a larger cohort and with a lower dose (1 mg/kg/qod) and shorter duration (one month) of corticosteroids. No significant increase in weight or height was observed after one month’s low dose prednisone, and neither severe adverse events were noted among all patients. All these results demonstrated that adjuvant prednisone therapy is safe for pediatric patients.
The retrospective observational design is subject to selection bias and limits the generalizability of the results. A further randomized controlled study of multimodal therapy for refractory IHs is worthwhile based on larger series.
In conclusion, the brief addition of low-dose oral prednisone is an effective and safe adjunctive treatment to beta-adrenergic receptor antagonists treatment in contributing to refractory IHs. The addition prednisone would not shorten the entire treatment duration. Both early administration and long enough duration would be necessary.
Acknowledgments
Funding: This study was financially supported by the grants of the National Natural Science Foundation of China (81771087 to Jiawei Zheng, 81901021 to Chao Liu) and Key Research and Development Program of Shandong (2019GSF108277 to Chao Liu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in accordance with the recommendations of Declaration of Helsinki. The protocol was approved by the Institute Review Board of Shanghai Ninth People’s Hospital (SH9H-2019-T272-1). The study outcomes will not affect the future management of the patients. All parents or guardian of participants of this study gave written informed consent in accordance with the Declaration of Helsinki for the participation in the study, and the publication of identifiable images.
References
- Zheng JW, Zhang L, Zhou Q, et al. A practical guide to treatment of infantile hemangiomas of the head and neck. Int J Clin Exp Med 2013;6:851-60. [PubMed]
- Goelz R, Poets CF. Incidence and treatment of infantile haemangioma in preterm infants. Arch Dis Child Fetal Neonatal Ed 2015;100:F85-91. [Crossref] [PubMed]
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51. [Crossref] [PubMed]
- Wu HW, Liu C, Wang X, et al. Topical Application of 0.5% Timolol Maleate Hydrogel for the Treatment of Superficial Infantile Hemangioma. Front Oncol 2017;7:137. [Crossref] [PubMed]
- Khan M, Boyce A, Prieto-Merino D, et al. The Role of Topical Timolol in the Treatment of Infantile Hemangiomas: A Systematic Review and Meta-analysis. Acta Derm Venereol 2017;97:1167-71. [Crossref] [PubMed]
- Ge J, Zheng J, Zhang L, et al. Oral propranolol combined with topical timolol for compound infantile hemangiomas: a retrospective study. Sci Rep 2016;6:19765. [Crossref] [PubMed]
- Zhang L, Wu HW, Yuan W, et al. Propranolol therapy for infantile hemangioma: our experience. Drug Des Devel Ther 2017;11:1401-8. [Crossref] [PubMed]
- Dong JY, Ning JX, Li K, et al. Analysis of factors affecting the therapeutic effect of propranolol for infantile haemangioma of the head and neck. Sci Rep 2017;7:342. [Crossref] [PubMed]
- Lie E, Puttgen KB. Corticosteroids as an adjunct to propranolol for infantile haemangiomas complicated by recalcitrant ulceration. Br J Dermatol 2017;176:1064-7. [Crossref] [PubMed]
- Charny JW, Moon AT, Treat JR. Scalp Infantile Hemangioma Complicated by Life-Threatening Bleeding. Pediatr Dermatol 2017;34:473-5. [Crossref] [PubMed]
- Aly MM, Hamza AF, Abdel Kader HM, et al. Therapeutic superiority of combined propranolol with short steroids course over propranolol monotherapy in infantile hemangioma. Eur J Pediatr 2015;174:1503-9. [Crossref] [PubMed]
- Anjum MZ, Pasha KH, Abbas SH, et al. The outcome of combination of low dose oral prednisolone with propranolol for the treatment of infantile haemangioma. Pak J Med Sci 2016;32:211-4. [PubMed]
- Nieuwenhuis K, de Laat PC, Janmohamed SR, et al. Infantile hemangioma: treatment with short course systemic corticosteroid therapy as an alternative for propranolol. Pediatr Dermatol 2013;30:64-70. [Crossref] [PubMed]
- Wong A, Hardy KL, Kitajewski AM, et al. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg 2012;130:1012-21. [Crossref] [PubMed]
- Munabi NC, England RW, Edwards AK, et al. Propranolol targets hemangioma stem cells via cAMP and mitogen-activated protein kinase regulation. Stem Cells Transl Med 2016;5:45-55. [Crossref] [PubMed]
- Ma X, Zhao T, Xiao Y, et al. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. Eur J Pediatr 2013;172:653-9. [Crossref] [PubMed]
- Kum JJ, Khan ZA. Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis. Pediatr Res 2014;75:381-8. [Crossref] [PubMed]
- Ji Y, Chen S, Li K, et al. The role of beta-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div 2013;8:1. [Crossref] [PubMed]
- Ma X, Zhao T, Ouyang T, et al. Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int J Clin Exp Pathol 2014;7:3809-17. [PubMed]
- Greene AK, Couto RA. Oral prednisolone for infantile hemangioma: efficacy and safety using a standardized treatment protocol. Plast Reconstr Surg 2011;128:743-52. [Crossref] [PubMed]


