
High TRIAP1 expression in penile carcinoma is associated with high risk of recurrence and poor survival
Introduction
Penile carcinoma (PeCa) is a rare genitourinary malignancy in developed countries, such as the United States or Europe, with an incidence of less than 1.00 per 100,000 men. However, in some regions of South America, Africa and Asia the incidence is markedly higher, accounting for up to 1–2% of malignancies in men (1,2). Phimosis with chronic inflammation, human papillomavirus (HPV) infection, poor hygiene, and smoking are the most common suggested prognostic factors (3). The majority of PeCa is squamous cell carcinoma (SCC), and penile cancer is known as an aggressive disease for early locoregional and angiolymphatic spread (4,5). Locally advanced and metastatic PeCa is associated with significant mortality and poor prognosis (6). A number of molecular markers have been evaluated to predict lymph node status as well as prognosis in PeCa. However, the clinical application of these biomarkers remains limited (7-9). Hence, there is a great need of effective biomarkers for disease progression of penile cancer.
Resisting cell death is one of the typical hallmarks of cancer (10). Proteins involved in mitochondrial network, which is at the core of programmed cell death or apoptosis, were considered to play a crucial role in the regulation of apoptotic pathway (11-13). TP53-regulated inhibitor of apoptosis 1 (TRIAP1), also known as p53 cell survival factor or p53CSV, does not share any similarity with XIAP, c-IAP, survivin or other members of the IAP family (14). TRIAP1 is a small 9-kDa protein of 76 amino acids long, and is transcriptionally activated by p53 (15). Its expression is induced by p53 following low levels of genotoxic stress (16). It has been reported that TRIAP1 inhibits apoptosis by binding heat shock protein 70 (HSP70) in the cytoplasm, or repressing p53 target gene cyclin-dependent kinase inhibitor 1 (p21) whose depletion slows down cell-cycle progression (16,17). TRIAP1 was also indicated to contribute to the resistance of apoptosis in a mitochondria-dependent manner in certain human malignancies (15,18). Besides, TRIAP1 was reported to represent a novel marker for drug resistance in breast cancer cell (19). However, the protein level of TRIAP1 in PeCa has not been determined, and its clinical value remains unknown for PeCa.
In present study, we found that the mRNA level of TRIAP1 is differentially expressed between PeCa tissues and normal penile tissues in the Gene Expression Omnibus (GEO, GSE57955) DataSets by bioinformatics analysis. Besides, we used immunohistochemistry (IHC) to validate the expression of TRIAP1 in PeCa tissues and normal tissues, and investigated the association between elevated TRIAP1 expression and the clinicopathological features of PeCa patients. TRIAP1 expression was considered a prognostic factor for local recurrence-free survival (RFS) of PeCa.
Methods
Patients and tissue samples
Informed consent was acquired from 57 patients undergoing radical penile surgery (partial or total penectomy) at the Department of Urology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China between Jan 2013 and Dec 2016. Our study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University. For immunohistochemical analysis, tissues from patients were assembled and stored at –80 °C. All PeCa samples were classified according to the 2017 Union for International Cancer Control (UICC) TNM staging as well as the 2016 World Health Organization/International Society of Urological Pathology (WHO/ISUP) classifications. All patients had no history of preoperative chemotherapy, or radiation therapy. The recorded clinical pathologic variables included age, stage, histologic grade, involvement of urethra, and lymph node metastasis.
Bioinformatics analysis
Data for differential genetic analysis were obtained from NCBI publicly available genomics database, Gene Expression Omnibus (GEO) DataSets (accession no. GSE57955, including 39 PeCa tissues and 5 normal penile tissues) (20). RNA was extracted from 39 fresh frozen PeCa samples (labeled with Cy3) and five normal glans (labeled with Cy5), and submitted to the Whole Human Genome 4 × 44K microarray platform GPL6480-9577 (Agilent Technologies) according to the manufacturer’s recommendations. The normal penile pool consisted of total RNA from five autopsy glands. The method for differential analysis was Bayes’ theorem based on the R limma package, using tissues from different locations (tumor or non-cancerous tissues) as variable for calculating differential expression. Genes with fold change (PeCa vs. normal penis tissue) >1.5 or <−1.5 and defined adjusted P value cutoff of <0.01 to be statistically significant. Log2 cy3/cy5 was used for the normalized gene expression of individual case.
Hematoxylin-eosin (HE) staining and IHC
The penile samples from patients were fixed in 10% buffered formalin. The sample tissues were cut into 3-µm thick sections and stained with HE staining according to standard protocols. Standard immunoperoxidase staining procedure was performed in observing TRIAP1 expression at protein level. Slides were dewaxed in xylene and rehydrated in different concentrations of ethanol, and then were immersed in 0.01 M citrate buffer to repair the antigen. Subsequently, slides were incubated with 3% hydrogen peroxide and placed at 37 °C for 15 minutes. Tissues were incubated with primary rabbit-anti-human monoclonal TRIAP1 antibody overnight. After washing with phosphate buffered saline, tissues were incubated with goat-anti-rabbit IgG as the secondary antibody. Then 3,3'-diaminobenzidine (DAB) was used to provide color development. Finally, tissues were counterstained with hematoxylin for 90 seconds, dehydrated in ethanol, and sealed with coverslips.
The expression of TRIAP1 at protein levels was assessed semi-quantitatively according to the sum score of the proportion of positive-stained cells and staining intensity. The proportion of positive stained cells was defined as follow: 0, less than 5% positive staining; 1, 5% to 50% positive staining; 2, more than 50% positive staining. The staining intensity was made as follows: 0, negative/weak positive; 1, moderate positive; and 2, strong positive. The sum of two parameters represented the expression levels: 0–2 was low expression; 3–4 was high expression (21). Independent score was estimated by two pathologists, the means of the scores was used as the final immunostaining score.
Statistical analysis
The SPSS Version 21.0 (SPSS, Chicago, IL, USA) and STATA Version 15.0 (StataCorp LLC, College Station, TX, USA) were used to analyze the data. The chi-square test was performed for variables. Kaplan-Meier (KM) survival analysis and the Cox proportional hazard model were conducted to determine the prognostic significance of TRIAP1 expression for the local RFS and overall survival (OS) for PeCa patients. Gene Expression Profiling Interactive Analysis (GEPIA) website (http://gepia.cancer-pku.cn/) based on TCGA Datasets was used for differential expression analysis of TRIAP1 in various human cancers (22). Two-sided P value of <0.05 were considered statistically significant.
Results
TRIAP1 mRNA level was elevated in PeCa tissues by bioinformatics analysis of the GEO Datasets
We used bioinformatics to analyze the differential expression of mRNAs in PeCa compared with normal tissues in GEO Dataset (GSE57955). We found 2,294 up-regulated genes and 3,582 down-regulated genes with statistical significance (P<0.01) in PeCa tissues (Figure 1A,B; supplementary file available at: http://fp.amegroups.cn/cms/atm.2019.06.47-1.pdf). Among these overexpressed genes, TRIAP1 mRNA expression level in PeCa tissues was higher than normal tissues with a two-fold change, along with P value of 0.007 as shown in Figure 1C. Therefore, in the clinical specimens of GEO Dataset, it was confirmed that TRIAP1 was significantly overexpressed in PeCa tissues when compared with normal tissues.
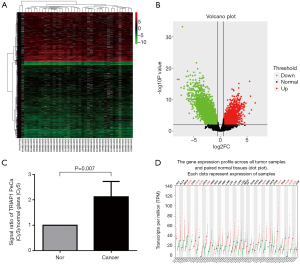
Besides, by GEPIA web-based tool (Figure 1D), we found that TRIAP1 expression is significantly up-regulated in some other human malignancies, including cervical SCC and endocervical adenocarcinoma (CESC), colon adenocarcinoma (COAD), Diffuse large B-cell lymphoma (DLBC), glioblastoma multiforme (GBM), rectum adenocarcinoma (READ), testicular germ cell tumors (TGCT), thymoma (THYM), uterine corpus endometrial carcinoma (UCEC) and uterine carcinosarcoma (UCS). Therefore, TRIAP1 was considered as a promising biomarker for various human cancers.
TRIAP1 protein level was overexpressed in PeCa tissues
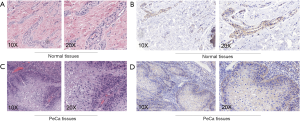
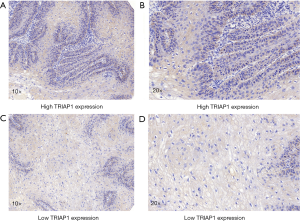
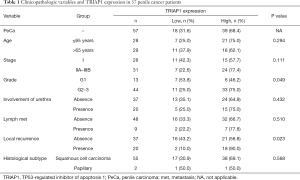
To validate the reliability of our bioinformatic data, the expression of TRIAP1 was determined in surgical specimens from 57 PeCa and 15 normal tissues in our hospital by immunohistochemical staining. As shown in Figure 2, TRIAP1 expression was mainly located in the cytoplasm of penile cancer cells. We found that TRIAP1 expression was elevated in PeCa, while was almost negatively expressed in normal tissues (Figure 2). As shown by using statistical analysis, the expression level of TRIAP1 in PeCa tissues was significantly higher than in normal tissues (P<0.001). Further, the expression of TRIAP1 in PeCa was classified as low or high in relation to median expression value as the cut-off point. We found TRIAP1 expression was high in 39 PeCa tissues and was relatively low in the other 18 tissues (Figure 3, Table 1).
Full table
The association between differential TRIAP1 expression and clinical characteristics in PeCa is also presented in Table 1. The median age of enrolled patients is 66.0 years old (range, 34.0–88.0). Based on patient data and immunohistochemical results from our clinical specimens, we found that strong intensity of TRIAP1 expression was significantly related with higher histological grade (P=0.049) and elevated local recurrence rate (P=0.023), suggesting TRIAP1 as a potential predictor in recurrence. However, no remarkable correlation was found between TRIAP1 expression and age (P=0.294), cancer stage (P=0.111), involvement of urethra (P=0.432) or lymph node metastasis (P=0.510).
TRIAP1 was correlated with poor prognosis in PeCa after radical penile surgery
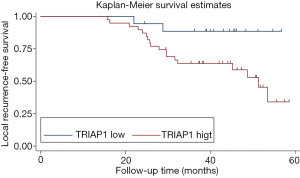
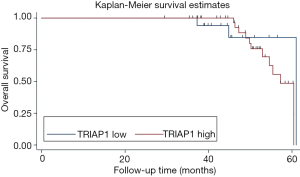
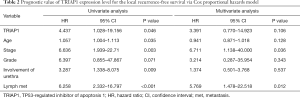
To investigate the significance of TRIAP1 expression level for prognosis of PeCa, we applied the KM curve analysis based on patient survival data (Figures 4,5). There were 20 out of 57 cases (35.1%) with local recurrence during the follow-up time of 24.4 months, and 14 out of 57 cases (24.6%) with death. The median time for local RFS and OS were 21.4 and 24.2 months, respectively. Regarding the relationship of TRIAP1 expression and prognosis, we found that PeCa patients with high TRIAP1 expression had a shorter local RFS time than those with low TRIAP1 expression (P value for log rank test =0.029) (Figure 4). Nevertheless, we didn’t find any significant difference between TRIAP1 expression and OS time in PeCa patients (P value for log rank test =0.501) (Figure 5). Univariate and multivariate Cox proportional hazards regression were further conducted to verify the clinical prognostic impact of TRIAP1. In univariate analysis, high TRIAP1 expression was identified to be a hazardous prognostic factor for local RFS [hazard ratio (HR) =4.437, P=0.046], as well as cancer stage (HR =6.636, P=0.003), histologic grade (HR =6.397, P=0.071), involvement of urethra (HR =3.287, P=0.009) and lymph node metastasis (HR =6.258, P<0.001) (Table 2). In multivariate analysis regarding local RFS, up-regulated TRIAP1 has also been indicated to predict a poor survival outcome but P value was not statistically significant (HR =3.391, P=0.106) (Table 2). Besides, cancer stage (HR =6.711, P=0.036) and lymph node metastasis (HR =5.769, P=0.012) were still identified as strong prognostic factors for local RFS by multivariate regression. In addition, both univariate analysis and multivariate analysis showed that TRIAP1 was not significantly related to OS in PeCa patients after radical penile surgery (Table 3).
Full table

Full table
Discussion
PeCa can be cured in 80% of cases if treated at an early stage, and has high mortality once metastatic spread has occurred (1). Immediate resection of occult lymph node metastases in patients with PeCa improves survival (23). When men with PeCa present for treatment, accurate staging is critical for prognostic and therapeutic information (24). Perineural and lymphatic invasion and grade are also prognostic predictors, and grading has been shown to be highly observer dependent (25). Sparse data link penile SCC to biological behavior have been reported. By detection of DNA sequence copy number alterations (CNAs) in penile SCC cases, lower copy and alteration numbers, particularly in the locus 8q24, have been reported to be linked to poor survival (26). Loss of heterozygosity around p16INK4A, which functions as a tumor-suppressor protein, and its promoter hypermethylation could be found more frequently in PeCa with metastasis (27). Besides, the expression of p53 in the primary PeCa specimen was reported to be negatively associated with cancer specific survival (28). However, there remains no effectual biomarker for PeCa. Consequently, potential prognostic factors of clinical value are in great demand, to improve the diagnostic and therapeutic understanding of PeCa.
To our knowledge, this is the first study on the role of TRIAP1 expression for the progression of PeCa. First, we found significant differential expression of TRIAP1 between PeCa tissues and normal tissues from GEO Datasets by bioinformatics analysis. In order to validate the reliability of bioinformatic data, we determined the TRIAP1 protein expression level by IHC using 57 surgical specimens of PeCa patients. Moreover, we applied GEPIA tool to investigate the TRIAP1 expression in various human malignancies, so as to verify the suitability of TRIAP1 as a tumor biomarker. Second, we assessed the correlation between the differential expression of TRIAP1 and clinical characteristics of PeCa patients. Interesting, high TRIAP1 expression was demonstrated to be significantly related to higher histological grade and elevated local recurrence rate. Third, our survival data showed that up-regulated TRIAP1 expression was a strong predictor for poor local RFS. However, the P value for multivariate analysis of local RFS lacked statistical significance, which might due to the enrolled patient number was not sufficient. Besides, limitation existed in our study. For instance, the size of enrolled cases from our center was not large enough due to the relative short follow-up time, which led to the insignificant prognostic value of differential TRIAP1 expression in both univariate and multivariate analyses of OS.
TRIAP1 contains a p53-binding site within its second exon and the reduction of expression by small interfering RNA enhanced apoptosis, whereas overexpression protected cells from apoptosis caused by DNA damage (16). TRIAP1 was found to be upregulated in at least 50% of the multiple myeloma patients, indicating that TRIAP1 was important for myeloma tumorigenesis and that could potentially be useful as therapeutic targets (29). Overexpression of TRIAP1 with miR-320b loss has also been reported to be associated with tumorigenesis and progression in nasopharyngeal carcinoma (NPC), which may represent prognostic markers and potential therapeutic targets for NPC treatment (15). Besides, it has been indicated that TRIAP1 is an estrogen-irresponsive gene, and up-regulation of TRIAP1 was found in drug-resistant breast cancer cells. Modulation of TRIAP1, either by overexpression or downregulation by RNA interference, changed cellular sensitivity of breast cancer cell to doxorubicin, thus confirming TRIAP1 as an effector of drug resistance (19). Moreover, knockdown of TRIAP1 was reported to result in a significant inhibition of cellular proliferation in AGS cells, but the partial silencing of TRIAP1 expression has significantly increased the invasion activity of colon cancer cells (30). Taken together, the role of TRIAP1 in cancers has not been fully clarified that further investigation is in great need. Meanwhile, our data indicated that there was an association between high TRIAP1 expression and advanced clinicopathological features and poor clinical outcome in PeCa patients, which improved the diagnostic and therapeutic understanding of PeCa. The total burden of TRIAP1 for pre PeCa patient is not expensive. Moreover, TRIAP1 evaluation is not complicated to perform according to standard protocols and is suitable to be popularized in every tertiary hospital. Hence, TRIAP1 could be a significant biomarker for prognosis of PeCa patients.
Conclusions
In summary, we demonstrated that TRIAP1 was up-regulated in PeCa patients by both GEO Datasets and clinical specimen. High TRIAP1 expression level was shown to be correlated with higher histological grade, and elevated TRIAP1 predicted shorter local RFS in patients who undergone radical penile surgery. Moreover, a randomized study with long-term follow-up is required to further verify TRIAP1 as a novel prognostic factor in PeCa patients.
Acknowledgments
We would like to thank ethics committee of the First Affiliated Hospital of Nanjing Medical University.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81570676, 81770751, 81100532, and 81470981).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (No. 1601140). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- Bethune G, Campbell J, Rocker A, et al. Clinical and pathologic factors of prognostic significance in penile squamous cell carcinoma in a North American population. Urology 2012;79:1092-7. [Crossref] [PubMed]
- Minhas S, Manseck A, Watya S, et al. Penile cancer--prevention and premalignant conditions. Urology 2010;76:S24-35. [Crossref] [PubMed]
- Ficarra V, Akduman B, Bouchot O, et al. Prognostic factors in penile cancer. Urology 2010;76:S66-73. [Crossref] [PubMed]
- Chipollini J, Chaing S, Azizi M, et al. Advances in Understanding of Penile Carcinogenesis: The Search for Actionable Targets. Int J Mol Sci 2017. [Crossref] [PubMed]
- Gupta S, Sonpavde G. Emerging Systemic Therapies for the Management of Penile Cancer. Urol Clin North Am 2016;43:481-91. [Crossref] [PubMed]
- Adimonye A, Stankiewicz E, Touche S, et al. The Prognostic Value of PIK3CA Copy Number Gain in Penile Cancer. Urology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Calmon MF, Tasso Mota M, Vassallo J, et al. Penile carcinoma: risk factors and molecular alterations. ScientificWorldJournal 2011;11:269-82. [Crossref] [PubMed]
- Rodney S, Feber A, Arya M, et al. Molecular markers in penile cancer. Curr Probl Cancer 2015;39:137-45. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Wang K, Zhou LY, Wang JX, et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun 2015;6:7619. [Crossref] [PubMed]
- Gao J, Liu M, Zou Y, et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol Rep 2015;34:3212-21. [Crossref] [PubMed]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 2008;22:1577-90. [Crossref] [PubMed]
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 2002;3:401-10. [Crossref] [PubMed]
- Li Y, Tang X, He Q, et al. Overexpression of Mitochondria Mediator Gene TRIAP1 by miR-320b Loss Is Associated with Progression in Nasopharyngeal Carcinoma. PLoS Genet 2016;12:e1006183. [Crossref] [PubMed]
- Park WR, Nakamura Y. p53CSV, a novel p53-inducible gene involved in the p53-dependent cell-survival pathway. Cancer Res 2005;65:1197-206. [Crossref] [PubMed]
- Andrysik Z, Kim J, Tan AC, et al. A genetic screen identifies TCF3/E2A and TRIAP1 as pathway-specific regulators of the cellular response to p53 activation. Cell Rep 2013;3:1346-54. [Crossref] [PubMed]
- Liu P, Qi X, Bian C, et al. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett 2017;13:4039-46. [Crossref] [PubMed]
- Adams C, Cazzanelli G, Rasul S, et al. Apoptosis inhibitor TRIAP1 is a novel effector of drug resistance. Oncol Rep 2015;34:415-22. [Crossref] [PubMed]
- Kuasne H, Colus IM, Busso AF, et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: uncovering new molecular markers. Clin Epigenetics 2015;7:46. [Crossref] [PubMed]
- Tan LD, Xu YY, Yu Y, et al. Serum HER2 level measured by dot blot: a valid and inexpensive assay for monitoring breast cancer progression. PLoS One 2011;6:e18764. [Crossref] [PubMed]
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98-102. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol 2005;173:816-9. [Crossref] [PubMed]
- Bloom JB, Stern M, Patel NH, et al. Detection of lymph node metastases in penile cancer. Transl Androl Urol 2018;7:879-86. [Crossref] [PubMed]
- Gunia S, Burger M, Hakenberg OW, et al. Inherent grading characteristics of individual pathologists contribute to clinically and prognostically relevant interobserver discordance concerning Broders' grading of penile squamous cell carcinomas. Urol Int 2013;90:207-13. [Crossref] [PubMed]
- Alves G, Heller A, Fiedler W, et al. Genetic imbalances in 26 cases of penile squamous cell carcinoma. Genes Chromosomes Cancer 2001;31:48-53. [Crossref] [PubMed]
- Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch 2011;458:221-9. [Crossref] [PubMed]
- Gunia S, Kakies C, Erbersdobler A, et al. Expression of p53, p21 and cyclin D1 in penile cancer: p53 predicts poor prognosis. J Clin Pathol 2012;65:232-6. [Crossref] [PubMed]
- Felix RS, Colleoni GW, Caballero OL, et al. SAGE analysis highlights the importance of p53csv, ddx5, mapkapk2 and ranbp2 to multiple myeloma tumorigenesis. Cancer Lett 2009;278:41-8. [Crossref] [PubMed]
- Yu K, Ganesan K, Tan LK, et al. A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers. PLoS Genet 2008;4:e1000129. [Crossref] [PubMed]


