Combining endoscopic ultrasound and tumor markers improves the diagnostic yield on the etiology of common bile duct dilation secondary to periampullary pathologies
Introduction
Unexplained common bile duct (CBD) dilatation may be caused by many etiologies, such as periampullary or pancreatic neoplasms, choledocholithiasis or inflammatory stenosis. Elder age and prior cholecystectomy are also related to CBD dilatation (1,2).
Abdominal ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are usually used first in the diagnostic work up of CBD dilatation, although their value is not high in many cases (3). Endoscopic retrograde cholangiopancreatography (ERCP) used to be the gold standard for the diagnosis of biliary diseases but is being gradually replaced by endoscopic ultrasonography (EUS), which has a similar diagnostic yield but not its well-known risks (4-6). Multiple studies have demonstrated the high sensitivity and specificity of EUS in the diagnosis of biliary diseases (7-9). But little is known about how to maximize the utility of EUS in differentiating malignant and benign CBD dilatation.
Herein we designed this study to evaluate the diagnostic yield of EUS on unexplained CBD dilatation, in conjunction with certain clinical factors including tumor markers, liver chemistry, symptoms, history of cholecystectomy and dilatation of the pancreatic duct (PD).
Methods
We retrospectively reviewed the EUS database of Zhongshan Hospital, Fudan University, between Jan 2016 and Jul 2017. Consecutive patients receiving EUS for dilated CBD who have nonconclusive prior imaging were included in this study. Patient demographics, symptoms, past history, laboratory results and imaging studies were recorded. The study was approved by Ethics Review Board of Zhongshan Hospital, Fudan University. The ID of the approval is B2019-101.
Patients included in this study were typically first seen by a surgeon for complaints such as jaundice and abdominal pain. Imaging studies, such as abdominal US, CT and MRI were ordered as appropriate as first-line diagnostic tools. When these studies failed to reach a diagnosis with high confidence or if malignancy was suspected, serology tumor markers were sent at the surgeon’s discretion. When the diagnosis was still nonconclusive, the patient was referred to EUS.
Although the diameter of CBD may increase with age, cholecystectomy or certain medication, 7 mm is usually considered the upper limit of normal (1,10-12). PD greater than 3 mm in diameter in the head or 2 mm in the body or tail on CT is generally considered dilated (13-15). These numbers are used to define CBD and PD dilation in this study.
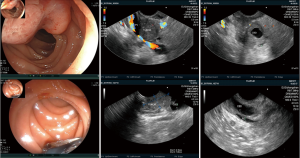
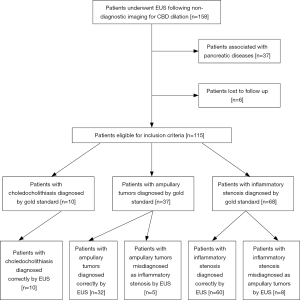
EUS was performed to examine the pancreas, CBD, duodenal papilla and ampulla of Vater with a linear echoendoscope (UCT-260, Olympus, Japan; SU-8000 or EG-530UT2, Fujifilm, Japan) by one experienced endosonographer (YQZ). EUS examination can be divided into two processes: direct observation to look for any obvious lesions around the ampulla by upper endoscopy and indirect observation to confirm the diagnoses by US. Typical EUS signs of periampullary tumors were shown in Figure 1.
The biological behavior of pancreatic tumors is completely different from periampullary tumors and patients with pancreatic diseases were excluded from the study to prevent bias. Thereafter we divided the causes of CBD dilatation into three categories: periampullary tumor, choledocholithiasis and inflammatory stenosis. A diagnosis of periampullary tumors was reached by histology (biopsy or surgical); a diagnosis of choledocholithiasis was reached by stone retrieval (endoscopic or surgical); a diagnosis of inflammatory stenosis was reached by histology (biopsy or surgical) and a lack of disease progression for at least 3 months.
Patients are included if: (I) known CBD dilation on more than one imaging; (II) no identifiable etiology; (III) single operator (YQZ). Patients were excluded if: (I) there is incomplete data; (II) malignancy other than periampullary tumors; (III) severe liver of kidney dysfunction or estimated life expectancy <6 months.
Statistical analyses were performed using the SPSS software (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as numbers and percentage. Student’s t-test, chi-square test and Fisher’s exact test were used as appropriate. Alpha was set at 0.05, two-sided.
Results
Patients and EUS findings
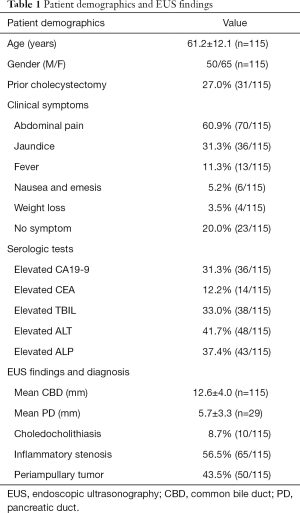
From January 2016 to July 2017, a total of 158 patients with CBD dilation underwent EUS following non-diagnostic imaging studies in our hospital. Thirty-seven patients with pancreatic diseases were excluded. Six patients had no records of treatment or follow-up and were also excluded from the study (Figure 2).
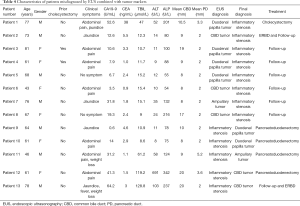
A total of 115 patients met the inclusion criteria and 65 (56.5%) were females. The mean age was 61.2±12.1 years (range, 25–85 years). The most common symptom was abdominal pain (70, 60.9%), followed by jaundice (36, 31.3%). Twenty-three patients (20.0%) were asymptomatic and had accidental finding of CBD dilation on imaging done for unrelated purposes. Abnormal tumor markers were found in 44 patients (38.3%) and abnormal liver chemistry was found in 59 patients (51.3%). Thirty-one patients (27.0%) had prior cholecystectomy. The mean diameter of CBD in all patients was 12.6±4.0 (range, 7.5–26.0) mm. Twenty-nine patients (25.2%) had PD dilatation, with a mean diameter of 5.7±3.3 (range, 2.9–20.0) mm. The details of patient demographics and EUS findings were shown in Table 1.
Full table
Diagnostic accuracy of EUS and imaging
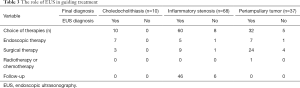
The diagnosis in all of the patients was confirmed by pathology and/ or a follow-up of no less than 3 months. Ten patients were confirmed to have choledocholithiasis, 37 periampullary tumors and 68 inflammatory stenosis (Table 2). All 10 cases of choledocholithiasis were correctly diagnosed by EUS and the sensitivity, specificity and accuracy of EUS for patients with choledocholithiasis were 100.0% (10/10), 100.0% (105/105) and 100.0% (115/115), respectively. Among the 37 patients with periampullary tumors, 32 were diagnosed correctly by EUS and 5 patients were misdiagnosed as inflammatory stenosis. The sensitivity, specificity and accuracy of EUS for patients with periampullary tumors were 86.5% (32/37), 89.7% (70/78) and 88.7% (102/115), respectively. Sixty-eight patients were confirmed to have inflammatory stenosis, of whom 60 were correctly diagnosed by EUS and 8 misdiagnosed as periampullary tumors. The sensitivity, specificity and accuracy of EUS for patients with inflammatory stenosis were 88.2% (60/68), 89.4% (42/47) and 88.7% (102/115), respectively. The overall diagnostic accuracy of EUS for CBD dilatation was 88.7% (102/115). As shown in Table 2, the overall diagnostic accuracy of US, CT, MRI and PET-CT for CBD dilatation were 64.1% (41/64), 66.2% (47/71), 67.0% (65/97) and 66.0% (33/50) and were significantly lower than that of EUS (P<0.001).

Full table
The role of EUS in guiding treatment
EUS correctly diagnosed all the 10 patients with choledocholithiasis, and finally 3 of them underwent choledocholithotomy with T-tube drainage while the other 7 underwent ERCP to remove the stones or place the stents. So far, no adverse events have been observed in all patients.
Among the 37 patients with periampullary tumors, 32 patients were diagnosed correctly by EUS, of which 21 patients underwent radical pancreaticoduodenectomy, 3 patients underwent cholecystectomy, CBD exploration with T-tube drainage according to their own conditions, 5 patients underwent endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR), 2 patients underwent endoscopic retrograde biliary drainage (ERBD) and 1 patient underwent chemotherapy. No adverse events had been observed except 2 patients, one died during the perioperative period of pancreaticoduodenectomy, and another one died 9 months after chemotherapy. Among the other 5 patients who were misdiagnosed as inflammatory stenosis by EUS, 4 of them were inclined to believe in what imaging or tumor markers suggested and underwent pancreaticoduodenectomy. And the other one, considering her own conditions and will, had a final choice of ERBD because of the discovery of tumor after 15 months follow-up time.
Sixty-eight patients were confirmed as inflammatory stenosis, and EUS correctly diagnosed 60 patients of them. Forty-six patients were followed up according to the diagnosis and 5 patients were confirmed to be benign by endoscopic brushing cytology, while 9 patients chose cholecystectomy, CBD exploration with T-tube drainage which also ruled out malignant diseases. 8 patients were misdiagnosed as periampullary tumor by EUS, of which 1 patient underwent ERBD, 1 patient underwent cholecystectomy and the other 6 patients chose to be followed up. The results of biopsy and follow-up confirmed the benignancy of the CBD. The mean follow-up time was 9.4±5.3 (3.0–20.0) months in 52 follow-up patients and no adverse events had been observed in all patients (Table 3).
Full table
Diagnostic accuracy of EUS combined with tumor markers
Although there are no specific tumor markers for periampullary tumors, CA19-9 and CEA are often elevated in patients with periampullary tumors and used as a diagnostic indicator in many literatures (16-20). In 115 patients, CEA and/or CA19-9 were elevated in 44 patients. 4 of the 10 patients diagnosed as choledocholithiasis, 20 of the 68 patients diagnosed as inflammatory stenosis, and 20 of the 37 patients diagnosed as periampullary tumors were associated with abnormal tumor markers. The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of tumor markers in the diagnosis of malignant CBD dilatation were 54.1% (20/37), 69.2% (54/78), 45.5% (20/44), 76.1% (54/71) and 64.3% (74/115), respectively.
In our study, we defined that only the diagnosis of tumor markers was consistent with EUS diagnosis results to identify the benign or malignant CBD dilatation. Seventy-nine patients were diagnosed as benign CBD dilatation by EUS combined with tumor markers, while 36 patients were diagnosed as malignant CBD dilatation. Two patients were misdiagnosed as periampullary tumors by EUS combined with tumor markers, which were proved to be inflammatory lesions by the pathology of surgical specimen and follow-up. Three patients were misdiagnosed as inflammatory stenosis by EUS combined with tumor markers, which were proved to be periampullary tumors by the pathology of surgical specimen. The details of all 13 patients misdiagnosed by EUS were shown in Table 4. The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of EUS combined with tumor markers in the diagnosis of malignant CBD dilatation were 91.9% (34/37), 97.4% (76/78), 94.4% (34/36), 96.2% (76/79) and 95.7% (110/115), respectively, which were significantly higher than that of EUS or tumor markers alone (P=0.049) (Table 2).
Full table
The effect of combination of other factors on the diagnosis of EUS
In clinical diagnosis, we usually use not only EUS, tumor markers and imaging to diagnose the CBD dilatation, but also the patient's clinical symptoms, prior history, serologic tests and other indicators. A significant increase in liver function is usually associated with malignant disease, but the specificity is not high. Some patients with severe choledocholithiasis or history of long-term drugs use may also have abnormal liver function.
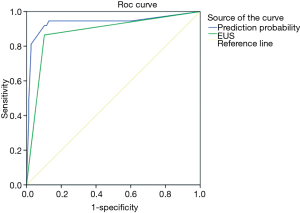
We can know from Table 5 that PD dilation (P=0.026) and weight loss (P=0.035) are significant for the identification of benign and malignant CBD dilatation. The receiver operating characteristics curve (ROC) plotted to predict malignancy disclose that the area under the curve (AUC) is 0.881 for EUS alone and 0.936 for EUS combined with PD dilation and weight loss, respectively (Figure 3). Therefore, the combination of EUS with PD dilation and weight loss is recommended in the diagnosis of malignant CBD dilatation.

Full table
Discussion
Most of unexplained CBD dilatation is related to periampullary diseases. Because of the special anatomy and function of ampulla, patients with periampullary diseases usually have earlier clinical symptoms and better prognosis than those with pancreatic diseases. Therefore, the crucial is to identify the cause as early as possible for guiding the early treatment. EUS can directly display the hierarchical structure of CBD and the histological features of the internal and surrounding organs, which is of great value in the diagnosis of the unexplained CBD dilatation. Our study evaluated the yield of EUS in differentiating malignant from benign CBD dilatation and what factors may affect the diagnosis of EUS which can guide the treatment of undetermined CBD dilatation.
Choledocholithiasis develops in approximately 10–20% of patients with gallbladder stones, while approximately 3–10% of patients undergoing cholecystectomy will have CBD stones (21). Cholangitis and obstructive jaundice usually occurs secondary to choledocholithiasis and severe patients even suffer from shock and coma. At present, imaging examinations are still the first choice for biliary and pancreatic diseases because of the convenience and non-invasiveness. In this study, the mean diameter of stones and CBD were 8.4±3.4 mm (range, 5.0–16.0 mm) and 12.0±4.8 mm (range, 8.0–24.0 mm). All patients had no PD dilation except one with the 4mm diameter of PD. The sensitivity of US, CT, MRI and PET-CT for patients with choledocholithiasis were 14.3% (1/7), 28.6% (2/7), 71.4% (5/7), 75.0% (3/4), which were significantly lower than that of EUS. US has been the traditional modality for evaluating cholecystolithiasis but is poor at detecting CBD stones (22-24) because it is easy to be disturbed by factors such as intestinal gas, obesity and the experience of the operator (25,26). The sensitivity and specificity of CT and MRI in detecting CBD stones are reported to be high (27-30), but their accuracy are reduced when a small stone (<5 mm) is present (27,31,32). The diameter of most of the patients misdiagnosed by CT and MRI in our study was less than 8mm, which may explain the low accuracy of CT and MRI. Although EUS is a relatively invasive examination, it can help us directly observe the lesion and surrounding tissue. It is reported that the sensitivity of EUS for diagnosing CBD stones is not affected by small size of stones (<5 mm), however, it is difficult to detect a stone impacted at the papilla (33). We diagnosed all 10 cases of choledocholithiasis by EUS, including a patient with a small stone close to the papilla. Other literatures reported that the diagnostic accuracy of choledocholithiasis was up to 93–99%, which was significantly higher than that of CT and MRI (34-36). Our research supported this view and helped to avoid the development of acute obstructive suppurative cholangitis. Seven patients received endoscopic treatment successfully and avoid surgical trauma. Therefore, when imaging examinations are negative, EUS is recommended to check for small CBD stones.
Periampullary tumors include neoplasias arising from lower CBD, ampulla of Vater, and periampullary duodenum. The initial evaluation of suspected periampullary tumors usually consists of imaging, but the accuracy varies greatly according to the tumor size (37). A total of 37 patients with periampullary tumors were included in our study, 18 of the tumors were found at ampulla of Vater and 19 were found at lower CBD. The accuracy of US, CT, MRI and PET-CT for differentiating between malignant and benign CBD dilation in this study were 70.2% (40/57), 70.3% (45/64), 66.7% (60/90), 65.2% (30/46), which were all significantly lower than what other literature reported (38-43). For the difficult cases of periampullary tumor, the signs of EUS are more obvious than those of imaging. The accuracy of EUS for periampullary tumors in this study was 88.7% (102/115), which was in accordance with the view that EUS was superior to imaging in the local assessment of periampullary tumors (44). Duraiswamy and Sreenarasimhaiah recommended EUS as the primary modality for evaluation of an abnormal bile duct because of its higher resolution and sensitivity (45). We are not sure about this even we take other disadvantages of imaging into consideration, such as expensiveness, allergic reaction and radioactivity. But when imaging can’t identify the cause, the discomfort and risk of the EUS examination should be forgotten and EUS should play a key role in guiding the diagnosis and treatment.
It is reported that the accuracy of US, CT and MRI is not high in patients with inflammatory stenosis, especially for the location and degree of the stenosis (46,47). It is considered that although there is no special EUS sign in patients with papillary inflammatory stenosis, EUS is still accurate in diagnosing inflammatory stenosis and localized pancreatic head inflammation (48). In our study, 8 patients were misdiagnosed as periampullary tumors, including 5 patients with papillary inflammatory stenosis. It was acceptable that only 1 misdiagnosed patient received unnecessary surgery. Some patients with periampullary diverticula or distortion of the duodenal bulb caused by duodenal ulcer disease can be hard to diagnose (34), but on the whole EUS can greatly improve the sensitivity and specificity of diagnosis of inflammatory stenosis. So if the imaging can not determine the cause of CBD dilatation, EUS should be the next step of diagnosis.
The high diagnostic accuracy of EUS has been reported by many literature and we are seeking to improve the role of EUS in the diagnosis of benign and malignant diseases. Owing to the low sensitivity, specificity, positive predictive value and negative predictive value, although tumor markers are widely used in clinical practice, they can only be initially screened for asymptomatic patients and have no definite diagnostic value for the periampullary tumor. Although the roles of CEA and CA 19-9 in periampullary cancers have not been clearly established, we still recommend the combination of EUS and tumor markers in the diagnosis of CBD dilatation. These two distinct methods are diagnosed by observing the lesion and detecting the carbohydrate antigens in the serum, respectively. False positive and false negative of tumor markers can be greatly reduced by EUS and the diagnostic rate of EUS in difficult cases can be improved by combining with tumor markers. Compared with the diagnosis of EUS alone in our study, the combined diagnosis prevented 6 patients with inflammatory stenosis from unnecessary surgery and made 2 patients with periampullary tumors succeed in prolonging their lives.
CBD dilatation with normal liver function is not always a benign condition (49). It is reported that PD dilatation is more likely to be associated with malignant disease and prior cholecystectomy is significantly associated with a negative EUS in patients with isolated CBD dilatation (50). Other literature also mentioned the effect of clinical symptoms on the diagnosis of EUS, such as patients with weight loss should pay more attention even that the EUS finding is negative (51). This study analyzed all the influencing factors and showed that EUS combined with tumor markers, PD dilation and weight loss can increase the diagnostic accuracy of EUS alone.
Because the EUS diagnosis is highly dependent on the experience of the endosonographers, all the patients in this study were examined and diagnosed by the same endosonographer to reduce the bias. However, the endosonographer may be disturbed by other imaging in difficult cases. Another limitation of our study was that the imagings were not reviewed by the same radiologist. And in our clinical work, surgeons may not follow the diagnostic results of EUS, but preferred imaging data which would lead to bias of selection.
In conclusion, the diagnosis of unexplained CBD dilation by EUS is far higher than that of imaging. EUS combined with tumor markers can be used clinically to identify the benign and malignant CBD dilatation. When patients are accompanied by PD dilation or weight loss, we should pay more attention to the possibility of malignant CBD dilatation.
Acknowledgments
Funding: Project supported by Shanghai Engineering and Research Center of Diagnostic and Therapeutic Endoscopy (No. 16DZ2280900), grant from Zhongshan Hospital (2015ZSYXGG14).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethics Review Board of Zhongshan Hospital, Fudan University. The ID of the approval is B2019-101. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Horrow MM. Ultrasound of the extrahepatic bile duct: issues of size. Ultrasound Q 2010;26:67-74. [Crossref] [PubMed]
- Senturk S, Miroglu TC, Bilici A, et al. Diameters of the common bile duct in adults and postcholecystectomy patients: a study with 64-slice CT. Eur J Radiol 2012;81:39-42. [Crossref] [PubMed]
- Holm AN, Gerke H. What should be done with a dilated bile duct?. Curr Gastroenterol Rep 2010;12:150-6. [Crossref] [PubMed]
- Adler DG, Jacobson BC, Davila RE, et al. ASGE guideline: complications of EUS. Gastrointest Endosc 2005;61:8-12. [Crossref] [PubMed]
- Tse F, Liu L, Barkun AN, et al. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008;67:235-44. [Crossref] [PubMed]
- Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc 2012;75:467-73. [Crossref] [PubMed]
- Fernández-Esparrach G, Ginès A, Sánchez M, et al. Comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the diagnosis of pancreatobiliary diseases: a prospective study. Am J Gastroenterol 2007;102:1632-9. [Crossref] [PubMed]
- Gan SI, Elizabeth R, Adler DG, et al. Role of EUS. Gastrointest Endosc 2007;66:425-34. [Crossref] [PubMed]
- Deerenberg EB, Poley JW, Hermans JJ, et al. Role of endoscopic ultrasonography in patients suspected of pancreatic cancer with negative helical MDCT scan. Dig Surg 2011;28:398-403. [Crossref] [PubMed]
- Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: how much do you want it to be? Am J Gastroenterol 2000;95:897-900. [Crossref] [PubMed]
- Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med 2000;19:727-30. [Crossref] [PubMed]
- Parulekar SG. Transabdominal sonography of bile ducts. Ultrasound Q 2002;18:187-202. [Crossref] [PubMed]
- Sahai AV. EUS and chronic pancreatitis. Gastrointest Endosc 2002;56:S76-81. [Crossref] [PubMed]
- Edge MDD, Hoteit M, Patel APP, et al. Clinical significance of main pancreatic duct dilation on computed tomography: single and double duct dilation. World J Gastroenterol 2007;13:1701-5. [Crossref] [PubMed]
- Hawes R, Fockens P. Endosonography. 2nd ed. Philadelphia, USA: Saunders, 2010.
- Kau SY, Shyr YM, Su CH, et al. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg 1999;188:415. [Crossref] [PubMed]
- Woo SM, Ryu JK, Lee SH, et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol 2007;14:3195-201. [Crossref] [PubMed]
- Tsukada K, Takada T, Miyazaki M, et al. Diagnosis of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 2008;15:31-40. [Crossref] [PubMed]
- Kurihara C, Yoshimi F, Sasaki K, et al. Clinical value of serum CA19-9 as a prognostic factor for the ampulla of Vater carcinoma. Hepatogastroenterology 2013;60:1588-91. [PubMed]
- Ahmad SR, Adler DG. Cancer of the ampulla of vater: current evaluation and therapy. Hosp Pract 1995;2014:45-61. [PubMed]
- Freitas ML, Bell RL, Duffy AJ. Choledocholithiasis: evolving standards for diagnosis and management. World J Gastroenterol 2006;12:3162e7.
- Nurman A. Imaging of common bile duct stones. Universa Medicina 2009;28(1).
- Mitchell SE, Clark RA. A comparison of computed tomography and sonography in choledocholithiasis. AJR Am J Roentgenol 1984;142:729. [Crossref] [PubMed]
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001;54:325. [Crossref] [PubMed]
- Taylor KJ, Rosenfield AT, Spiro HM. Diagnostic accuracy of gray scale ultrasongraphy for the jaundiced patient. A report of 275 cases. Arch Intern Med 1979;139:60-3. [Crossref] [PubMed]
- Berberat P, Friess H, Kashiwagi M, et al. Diagnosis and staging of pancreatic cancer by positron emission tomography. World J Surg 1999;23:882-7. [Crossref] [PubMed]
- Tseng CW, Chen CC, Chen TS, et al. Can computed tomography with coronal reconstruction improve the diagnosis of choledocholithiasis? J Gastroenterol Hepatol 2008;23:1586-9. [Crossref] [PubMed]
- Lee JK, Kim TK, Byun JH, et al. Diagnosis of intrahepatic and common duct stones: Combined unenhanced and contrast-enhanced helical CT in 1090 patients. Abdom Imaging 2006;31:425-32. [Crossref] [PubMed]
- Verma D, Kapadia A, Eisen GM, et al. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006;64:248-54. [Crossref] [PubMed]
- Romagnuolo J, Bardou M, Rahme E, et al. Magnetic Resonance Cholangiopancreatography: A Meta-Analysis of Test Performance in Suspected Biliary Disease. Ann Intern Med 2003;139:547. [Crossref] [PubMed]
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005;54:271. [Crossref] [PubMed]
- Isherwood J, Garcea G, Williams R, et al. Serology and ultrasound for diagnosis of choledocholithiasis. Ann R Coll Surg Engl 2014;96:224-8. [Crossref] [PubMed]
- Chen CC. The efficacy of endoscopic ultrasound for the diagnosis of common bile duct stones as compared to CT, MRCP, and ERCP. J Chin Med Assoc 2012;75:301-2. [Crossref] [PubMed]
- Prachayakul V, Aswakul P, Bhunthumkomol P, et al. Diagnostic yield of endoscopic ultrasonography in patients with intermediate or high likelihood of choledocholithiasis: a retrospective study from one university-based endoscopy center. BMC Gastroenterol 2014;14:165. [Crossref] [PubMed]
- Morris S, Gurusamy KS, Sheringham J, et al. Cost-Effectiveness Analysis of Endoscopic Ultrasound versus Magnetic Resonance Cholangiopancreatography in Patients with Suspected Common Bile Duct Stones. PLoS One 2015;10:e0121699. [Crossref] [PubMed]
- Liu CL, Lo CM, Chan JK, et al. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc 2000;51:28-32. [Crossref] [PubMed]
- Snady H, Avram Cooperman M, Jerome Siegel M. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc 1992;38:27-34. [Crossref] [PubMed]
- Andersen HB. CT for assessment of pancreatic and periampullary cancer. Acta Radiol 1993;34:569-72. [Crossref] [PubMed]
- Andersson M, Kostic S, Johansson M, et al. MRI combined with MR cholangiopancreatography versus helical CT in the evaluation of patients with suspected periampullary tumors: a prospective comparative study. Acta Radiol 2005;46:16-27. [Crossref] [PubMed]
- Rösch T, Meining A, Frühmorgen S, et al. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc 2002;55:870-6. [Crossref] [PubMed]
- Rösch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology 1992;102:188. [Crossref] [PubMed]
- Raj P, Kaman L, Singh R, et al. Sensitivity and specificity of FDG PET-CT scan in detecting lymph node metastasis in operable periampullary tumours in correlation with the final histopathology after curative surgery. Updates Surg 2013;65:103-7. [Crossref] [PubMed]
- Verma A, Shukla S, Verma N. Diagnosis, Preoperative Evaluation, and Assessment of Resectability of Pancreatic and Periampullary Cancer. Indian J Surg 2015;77:362-70. [Crossref] [PubMed]
- Chen CH, Tseng LJ, Yang CC, et al. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. J Clin Ultrasound 2001;29:313. [Crossref] [PubMed]
- Duraiswamy S, Sreenarasimhaiah J. T1479: Efficacy of Endoscopic Ultrasound in the Evaluation of Bile Duct Abnormalities: Comparison With CT, MRI, and ERCP. Gastrointest Endosc 2010;71:AB287-8. [Crossref]
- Meister T, Heinzow HS, Woestmeyer C, et al. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J Gastroenterol 2013;19:874-81. [Crossref] [PubMed]
- Tantau M, Pop T, Badea R, et al. Intraductal ultrasonography for the assessment of preoperative biliary and pancreatic strictures. J Gastrointestin Liver Dis 2008;17:217. [PubMed]
- Hao F, Qin MF, Li N, et al. Endoscopic ultrasonography for inflammatory distal biliary stricture: Analysis of 165 cases. World Chin J Dig 2013;21(28).
- Bruno M, Brizzi RF, Mezzabotta L, et al. Unexplained common bile duct dilatation with normal serum liver enzymes: diagnostic yield of endoscopic ultrasound and follow-up of this condition. J Clin Gastroenterol 2014;48:e67-70. [Crossref] [PubMed]
- Oppong KW, Mitra V, Scott J, et al. Endoscopic ultrasound in patients with normal liver blood tests and unexplained dilatation of common bile duct and or pancreatic duct. Scand J Gastroenterol 2014;49:473. [Crossref] [PubMed]
- Chen CH, Yang CC, Yeh YH. For Biliary Dilatation, a Negative Endosonography Needs Additional Image Studies in Weight Loss Suggesting Malignancy. Dig Dis Sci 2013;58:2345-52. [Crossref] [PubMed]