
The association between post-procedural oral hydration and risk of contrast-induced acute kidney injury among ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention
Introduction
CI-AKI was a serious complication of angiographic procedures and can constitute up to 10% of hospital-acquired AKI (1). Severe acute kidney injury required renal replacement therapy and results in increased morbidity and mortality, prolonged hospitalization, and overall increased healthcare costs (2-4). Of concern, 14.5% of the patients undergoing cardiac catheterization developed CI-AKI; reports had illustrated 50% incidence in high-risk patients, such as patients with STEMI (5-8). What was worse, CI-AKI was associated with poorer outcomes, even in STEMI patients without impaired left ventricular ejection fraction (LVEF) and with normal renal function (9,10).
According to the 2018 ESC/EACTS Guidelines on myocardial revascularization, prophylactic hydration was recommended in high-risk patients (11). However, conflicting results had arisen regarding whether oral hydration prevents CI-AKI. The optimal evidence of oral hydration strategy had not been well-established in a high-risk population such as STEMI patients undergoing primary PCI.
Given the data, this study was designed to evaluate the association between post-procedural oral hydration and risk of contrast-induced acute kidney injury among STEMI patients undergoing primary PCI. To the best of our knowledge, this was the first attempt to investigate the association of oral hydration and CI-AKI in STEMI patients undergoing primary PCI.
Methods
Study design and population
This was a prospective, observational study. 308 Patients aged ≥18 years with STEMI undergoing primary PCI and provided written informed consent were consecutively enrolled from March 2012 till December 2013. Exclusion criteria included (12): (I) pregnancy, (II) lactation, (III) intravascular administration of contrast medium within the last 7 or 3 days postoperatively, (IV) lack of use of low-osmolarity contrast agents, (V) missing postoperative oral hydration volume records, (VI) cardiovascular surgery or endovascular repair, (VII) end-stage renal disease or renal replacement, (VIII) missing preoperative or postoperative creatinine data, (IX) malignancy, (X) missing weight data. All data in the study was collected following approval by the ethics committees (approval number: GDREC2010112H).
Data collection
All eligible participants received peri-procedural intravenous hydration of routine regimens. Oral hydration began at an unlimited rate until 24 h after the coronary procedure. By monitoring the fluid status, the rate and duration of oral intake, as well as the use of diuretics, were based on the clinical evaluation of heart function and at the discretion of the cardiologists. Additionally, oral hydration volume/weight (OHV/W) ratios were calculated. Hemodynamic data were obtained in various clinical settings to assess volume status and guide medical therapy, including the administration of intravenous fluids.
The primary outcome measure was CI-AKI, defined as a 25% or 0.5 mg/dL increase in serum creatinine from baseline during the first 48–72 hours post-procedure. Secondary endpoints were the stroke, acute heart failure, in-hospital death, replacement therapy during hospitalization.
All data were collected using standardized electronic case report forms. At the time of entry, the data management team (of Guangdong Provincial People’s Hospital) performed consistency checks and issues electronic data clarification forms on discrepant data. An independent data monitoring committee was responsible for the review of the ongoing safety of patients enrolled in the study.
Statistical analysis
Continuous variables were outlined by means ± standard deviation or medians and interquartile ranges according to distribution. Categorical variables were presented as frequencies and percentages. OHV/W ratios were calculated and grouped according to 12 mL/kg. For continuous variables, the groups were compared using Student t-test or Wilcoxon rank sum test according to distribution. Chi-square and Fisher exact tests were used to compare categorical variables. The odds ratios (OR), as well as 95% confidence interval (CI), of CI-AKI for different OHV/W ratios, were estimated using multivariate logistic regression analyses. Multivariable logistic regression models were developed to adjust for clinical characteristics (e.g., age, sex, baseline serum creatinine, diabetes mellitus, congestive heart failure, intravenous hydration and use of diuretics). A 2-tailed P value <0.05 was considered statistically significant.
All statistical analyses were performed using SAS version 9.4 or later (SAS Institute, Cary, NC, USA) and R software (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Finally, 308 patients with STEMI underwent primary PCI without exclusion criteria were included in the analysis. The patients were divided into two groups: the adequate group (Group 1) with an OHV/W ratio ≥12 mL/kg, and the inadequate group (Group 2) with an OHV/W ratio <12 mL/kg. Post-procedural prophylactic oral hydration was implemented in 90.91% (280/308) of STEMI patients undergoing primary PCI. There were no differences in terms of sex, age, weight, index systolic blood pressure, diastolic blood pressure, heart rate, LVEF, anemia, diabetes mellitus, hypertension, hyperlipidemia, prior myocardial infarction, a history of coronary artery bypass grafting, contrast volume, number of implanted stents, as well as medications such as angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, β-blocker, and calcium channel blocker. However, patients in Group 1 were higher in creatinine clearance and lower in baseline serum creatinine, blood urea nitrogen as well as contrast volume/creatinine clearance ratio than Group 2. Furthermore, those in Group 1 had lower Mehran score, and received less intravenous hydration volume but more OHV. They were less likely to present with preprocedural hypotension, congestive heart failure, and to receive diuretics than those receiving inadequate oral hydration (Table 1).
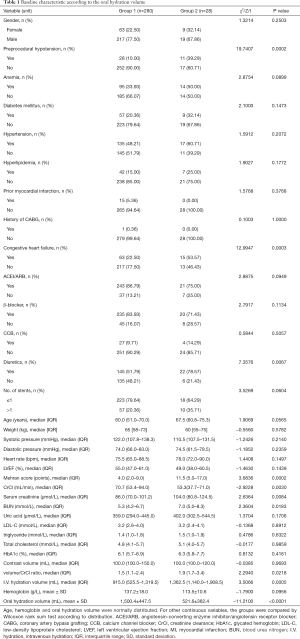
Full table
OHV/W for predicting CI-AKI
The incidence of CI-AKI was much higher in Group 2 than Group 1 (53.57% vs. 21.79%, respectively, P=0.0002). Moreover, patients in Group 2 were more likely to have a stroke (10.71% vs. 1.08%, P=0.0113), acute heart failure (39.29% vs. 7.89%, P<0.0001), renal replacement therapy (25.00% vs. 2.14%, P<0.0001), and in-hospital death (39.29% vs. 2.14%, P<0.0001) (Table 2). To investigate the association between OHV/W ratio and CI-AKI, a multivariate logistic regression analysis was performed. After adjusting for age, female, baseline serum creatinine, diabetes mellitus, congestive heart failure, intravenous hydration volume, and use of diuretics, multivariate logistic regression analysis indicated that postprocedural OHV/W ratio ≥12 mL/kg was an independent protective factor associated with CI-AKI (odd ratio, 0.349; 95% confidence interval, 0.147–0.828; P=0.0170) (Table 3). Forest plot showed the association between the postprocedural OHV/W ratio and the adjusted risk of CI-AKI (Figure 1).
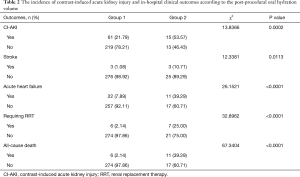
Full table

Full table
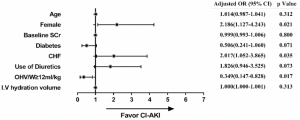
Discussion
The incidence of CI-AKI was much higher in the inadequate oral hydration group than the adequate group. Multivariate logistic regression showed inadequate oral hydration was the independent predictor associated with CI-AKI. Our study determined the association of post-procedural adequate oral hydration on CI-AKI following primary PCI, which was a potential strategy for CI-AKI prevention among patients with STEMI at very high risk. This was the first attempt to investigate the association of oral hydration and CI-AKI in STEMI patients undergoing primary PCI.
Patients with STEMI were likely to present with hypotension or even shock, contrast media, and it was often impossible to start renal prophylactic therapy, which was associated with an increased risk of CI-AKI (13). Despite a considerable prevalence of risk factors, including reduced LVEF, renal dysfunction, and diabetes mellitus in patients needing adequate hydration (14-16), cardiologists in previous studies were primarily concerned with rapid revascularization for occluded culprit arteries instead of adequate pre-procedural hydration to prevent CI-AKI; this was related to a lack of information regarding baseline renal function and related medical history. Therefore, post-procedural oral hydration might be more suitable for CI-AKI prevention in patients with STEMI.
In our study, baseline blood pressure, left ventricular ejection fraction were similar in both the inadequate and the adequate oral hydration group. However, patients were more likely to have preprocedural hypotension and congestive heart failure. This potentially explained the reason of the higher Mehran score in the inadequate oral hydration group and results in higher risk of CI-AKI. Later, a multivariate logistic regression model was built up to confirm the associations between CI-AKI and the 24-hour postprocedural OHV.
In 1998, Taylor et al. (16) reported that oral hydration was equally effective as intravenous hydration. In 2006, Dussol et al. (17) carried out a small-sample, randomized controlled trial and demonstrated oral saline hydration was as efficient as intravenous saline hydration for the prevention of CI-AKI in patients with chronic kidney diseases. In the meantime, it showed furosemide and theophylline were not protective. Four meta-analyses had been published so far, which included 4–8 randomized controlled trials (18-21). Zhang et al. (21) conducted a meta-analysis demonstrating that oral hydration was as effective as intravenous fluid hydration regimens in the prevention of CI-AKI (odds ratio: 0.73; 95% CI: 0.36–1.47; P>0.05).
Previous studies were conducted on relatively low-risk patients, including those subjects undergoing intravenous radiographic procedures or elective percutaneous coronary intervention. The frequency of risk factors was merely reported, and some studies excluded patients with chronic kidney disease, CHF, or systolic dysfunction with a lower proportion of diabetic patients. Moreover, the oral hydration protocol varied greatly, with no two trials having a similar oral regimen, and none of them was adjusted by patients’ weight.
It was reported that the incidence was <2% in the general population but was up to 20–30% in high-risk populations with congestive heart failure, chronic kidney disease, diabetes mellitus, and anaemia (1). For inpatient settings or individuals who required emergent coronary angiography or radiological procedures with contrast exposures, intravenous hydration had been studied and used as first-line treatment for prevention of CI-AKI (11). However, there was no consensus regarding the mode of administration. In modern medicine, with an evolving number of diagnostic studies that depended on iodinated contrast along with an increasing number of complex high-risk patients, costs of hospitalizations and nursing care were growing. Previous hydration strategies had not been investigated in STEMI patients. Therefore, oral hydration, which was considered safe and effective in low-risk patients, should be investigated in patients with STEMI undergoing primary PCI.
Limitations
Our current analysis was subject to the following limitations. First, it was less sensitive than defined as a ≥0.5 mg/dL increase in serum creatinine, because it recognized less selectively those patients with a higher risk of mortality and morbidity. Second, all participants received routine intravenous hydration (500 mL). Haemodilution could reduce serum creatinine, and cumulative daily fluid balance (input/output) directly affected the concentration (i.e., dilution) of serum creatinine. In our study, post-procedural daily fluid balance (input/output) was recorded to estimate the change in renal function to reduce the influence of haemodilution. Third, the fact that post-procedure serum creatinine measurements were not random but standardized at 48 hours might suggest that delayed-onset elevation of serum creatinine (>48 hours) could be overlooked. Finally, this observational analysis was not able to conclude a causal relationship. On the basis of the above limitations, future large-sample, well-designed randomized controlled trials were required to confirm and update the findings of our study. However, to the best of our knowledge, this was the first attempt to investigate the association of oral hydration and CI-AKI in STEMI patients undergoing primary PCI.
Conclusions
Oral hydration had a practical value in daily life. It was easy to administer, allowed better use of hospital resources due to shorter hospital stays, did not require intravascular cannulation, was less expensive, and was more comfortable for the patient. Our study determined the association between post-procedural adequate oral hydration and the decreasing incidence of CI-AKI following primary PCI, which was a potential strategy for CI-AKI prevention among patients with STEMI at very high risk.
Acknowledgments
Funding: This study was supported by grants from the Science and Technology Planning Project of Guangdong Province (No. 2014B070706010), the Guangdong Scientific Research Foundation (No. A2016510), the National Science Foundation for Young Scientist of China (No. 81500520), the Progress in Science and Technology Project of Guangdong Province (No. 2015A030302037), the Guangdong General Hospital Clinical Research Fund (No. GSIC20140526) and Beijing Lisheng Cardiovascular pilot Foundation (No. LHJJ201612127).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All data in the study was collected following approval by the ethics committees (approval number: GDREC2010112H) and was registered on the ClinicalTrials.gov with the registration ID NCT01400295.
References
- Singri N, Ahya SN, Levin ML. Acute renal failure. JAMA 2003;289:747-51. [Crossref] [PubMed]
- McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 1997;103:368-75. [Crossref] [PubMed]
- Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 2006;113:1799-806. [Crossref] [PubMed]
- James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123:409-16. [Crossref] [PubMed]
- Sadeghi HM, Stone GW, Grines CL, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation 2003;108:2769-75. [Crossref] [PubMed]
- Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J 2014;35:1533-40. [Crossref] [PubMed]
- Gupta R, Birnbaum Y, Uretsky BF. The renal patient with coronary artery disease: current concepts and dilemmas. J Am Coll Cardiol 2004;44:1343-53. [PubMed]
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275:1489-94. [Crossref] [PubMed]
- Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004;44:1780-5. [Crossref] [PubMed]
- Pyxaras SA, Sinagra G, Mangiacapra F, et al. Contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention without acute left ventricular ejection fraction impairment. Am J Cardiol 2013;111:684-8. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527-41. [Crossref] [PubMed]
- Zoungas S, Ninomiya T, Huxley R, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med 2009;151:631-8. [Crossref] [PubMed]
- Sgura FA, Bertelli L, Monopoli D, et al. Mehran contrast-induced nephropathy risk score predicts short- and long-term clinical outcomes in patients with ST-elevation-myocardial infarction. Circ Cardiovasc Interv 2010;3:491-8. [Crossref] [PubMed]
- Oduncu V, Erkol A, Karabay CY, et al. Relation of the severity of contrast induced nephropathy to SYNTAX score and long term prognosis in patients treated with primary percutaneous coronary intervention. Int J Cardiol 2013;168:3480-5. [Crossref] [PubMed]
- Taylor AJ, Hotchkiss D, Morse RW, et al. PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest 1998;114:1570-4. [Crossref] [PubMed]
- Dussol B, Morange S, Loundoun A, et al. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant 2006;21:2120-6. [Crossref] [PubMed]
- Cheungpasitporn W, Thongprayoon C, Brabec BA, et al. Oral hydration for prevention of contrast-induced acute kidney injury in elective radiological procedures: a systematic review and meta-analysis of randomized controlled trials. N Am J Med Sci 2014;6:618-24. [Crossref] [PubMed]
- Hiremath S, Akbari A, Shabana W, et al. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLoS One 2013;8:e60009. [Crossref] [PubMed]
- Agarwal SK, Mohareb S, Patel A, et al. Systematic oral hydration with water is similar to parenteral hydration for prevention of contrast-induced nephropathy: an updated meta-analysis of randomised clinical data. Open Heart 2015;2:e000317. [Crossref] [PubMed]
- Zhang W, Zhang J, Yang B, et al. Effectiveness of oral hydration in preventing contrast-induced acute kidney injury in patients undergoing coronary angiography or intervention: a pairwise and network meta-analysis. Coron Artery Dis 2018;29:286-93. [Crossref] [PubMed]


