Immunity in cancer and atherosclerosis
Introduction
For decades, cancer and cardiovascular diseases were considered non-communicable. Both types of diseases have characteristics of immune reactions, which are principally identical, but differing in important aspects. The aim of this communication is to highlight new approaches to immune processes involved in both types of diseases.
Cancer
The hypothesis describing the involvement of immune processes in development of cancer is rather old. Paul Ehrlich suggested that immunity can somehow protect against cancer cells (1-3). He based his assumption on the fact that cancer is found mostly in long-lived species. Our knowledge of the immune system structure and mechanisms in his time was extremely limited, so it was virtually impossible to experimentally confirm his prediction.
Immunosurveillance
The immunosurveillance hypothesis appeared approximately 50 years later (4,5). It was proposed that lymphocytes act as sentinels in recognizing and eliminating continuously arising, nascent transformed cells. It was experimentally validated when the discovery of specific cancer antigens (6) confirmed involvement of immunity in the development of cancer. Subsequent experiments were, however, contradictory and did not confirm this hypothesis (7,8). New studies have found that cancers can develop spontaneously. Furthermore, it can be induced by carcinogenic chemicals in both immunocompetent and partly or even fully immunodeficient athymic mice. These findings led many immunologists to reject this hypothesis. Some authors argued that cancer cells do not send out adequate signals about danger, thus, no immune response is induced (9). Other authors believe the immune system ignores, or at least tolerates, cells developing from normal cells (10) or that constant activation of the proinflammatory branch of natural (innate) immunity inhibits its immunoprotective role. Subsequently, this makes both tumor transformation and development easier (11,12).
In the early 1990s, we witnessed the rebirth of the immunosurveillance hypothesis. The main reasons for the change were better models of immunodeficient mice and findings of the role of interferon-γ (IFN-γ)—in rejection of transplanted tumors (13). The direct involvement of immune reaction in inhibition, or at least suppression of cancer growth resulting in death of cancer cells, was subsequently confirmed by many authors; for review, see Vesely et al. (14).
Double role of immunity
It is established that the immune system destroys viruses causing cancer growth. At the same time, the immune system inhibits formation and development of inflammatory microenvironment, which would help to eliminate invading pathogens. Therefore, the final consequence can be facilitation of cancer growth. It is clear that in the case of cancer origin, development and spreading, the immune system plays a dual, rather opposite, role. The immune system not only destroys tumor cells, blocking subsequent proliferation of cancer cells, but it also helps their multiplication and spreading. The unwanted consequence of this immune selection is the survival of the most resistant cancer cells. In the center of the tumor a specific microenvironment occurs, helping the cells to not only survive and further divide, but to spread to other organs and tissues.
Immunoediting
The dual role of immunity in malign processes is called tumor immunoediting (15,16). This concept describes reciprocal interaction of tumor cells with cells and humoral factors of the immune system. Less immunogenic clones of tumor cells are selected during immunoediting. This means the clones of cells expressing antigen do not cause immune response. Interaction of tumors with the immune system can be divided into three main phases: elimination, balance and escape.
Elimination phase
This phase represents the well-known immunosurveillance. It includes cell and humoral factors of both natural and adaptive immune response, which controls the growth of tumors and results in destruction (i.e., elimination). The main effector cells are natural killer (NK) cells and T lymphocytes, which are activated by pro-inflammatory cytokines produced by macrophages, and malign and stromal cells. NK cells infiltrate tumor and the tumor cells are killed by various cytokines such as IL-12 and IFN-γ (17). The elimination phase can be further subdivided into four subphases: (I) complex processes of immune recognition; (II) production of tumor cell-killing cytokines; (III) production of reactive oxygen and nitrogen species; and (IV) differentiation of cytotoxic T cells [natural killer T (NKT) cells]. When all tumors are not killed, the tumor will convert into a balanced phase.
Balanced phase
This phase is the longest interval of immunoediting. Tumor cells, which in the previous phase escaped elimination, express nonimmunogenic phenotype. However, they are genetically unstable and are susceptible to multiple mutations and to changes caused by epigenetic mechanisms. The immune system keeps malignant tissue in a steady homeostatic state. Genetic and epigenetic changes result in production of cell clones, which are not only a little immunogenic, but are also resistant against cellular and humoral immune attacks. The numbers of malignant cells are relatively small and clinically virtually undetectable. However, selection still occurs, although slowly. This selection, which can take years, still results in the formation of new variants. They can be fully resistant to any immune actions and slowly move into the last phase of the immunoediting; i.e., phase of escape. The existence of the balanced phase is confirmed by cases when a recipient obtains an organ from a donor with cured cancer, but the cancer develops again in the same transplanted organ (17).
Escape phase
This phase labels the stage when a new microenvironment is formed inside the malignant tissue. This microenvironment is immunosuppressive and allows uncontrollable growth of tumor cells, which is already clinically noticeable. Cancer cells express signaling factors which are involved in inflammation processes and during autoimmune response. Such a signaling molecules represents e.g., programmed death-ligand 1 (PD-L1) binding to the immune checkpoint molecule, the programmed cell death protein (PD-1) on CD8+ T cells. Then, ligation of PD-L1 to PD-1 decreases function. Binding of tumor-derived PD-L1 to PD-1 decreases anticancerous activity of T cells and thus the host immune response against tumor (18,19).
In addition, cells such as macrophages, NK, NKT, neutrophils, dendritic cells, and vascular endothelial cells secreting various chemokines and growth factors, also appear inside tumors (18). Immune reaction of the patient is focused toward several more or less potentially malignant cell variants, but spares resistant cells selected during the balanced phase. Additional mechanisms allowing tumor cells to escape the immune reaction exist and include loss of MHC I antigens, observed in up to 90% of all tumors (19). On the other hand, increased expression of neoclassic MHC I (HLA-E, HLA-F, HLA-G) leads to suppression of NK cell activity (20). Tumor microenvironment is immunologically highly complex and, to an extent, mostly immunosuppressive, but paradoxically offers sophisticated defense to malignant cells. As soon as the tumor reaches this phase, it is necessary to use fundamentally different procedures for a final elimination; i.e., surgery, chemotherapy and/or irradiation (21).
Atherosclerosis
Cardiovascular diseases are the most common cause of death worldwide. By 2030, the number of deaths is expected to reach 30 million (22). Atherosclerosis is one of the main causes of cardiovascular diseases. Atherosclerosis represents a multiphase pathological process characterized by activation of endothelial cells with subsequent expression of adhesion molecules. Current opinions believe that arteries represent tertiary lymphatic organs with variable cellular complexity, producing several immunocompetent factors. In these functions, artery tertiary lymphoid organs (ATLO) are similar to secondary lymphoid tissues (23). ATLO emerge during nonresolving peripheral inflammation, but their impact on disease progression is not fully known.
Inflammation
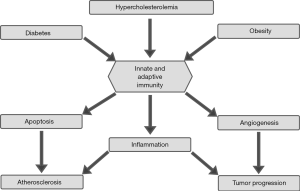
Contrary to carcinogenesis, which is clearly and markedly subdivided and immune reactions play a dual role, atherosclerosis represent mostly stratified inflammation with the major role played by monocytes and macrophages. Inflammation is a physiological response to various stimuli by invading microbes, irritants, or injury. The main function of inflammation is to eliminate the initial cause, to clear out necrotic cells and tissues damaged from either the original attack or from the inflammatory process, and to start tissue repair. It has long been a well-known symptom of various infectious diseases, but current molecular and epidemiological research increasingly supports the idea that it is also intimately linked with a broad range of noninfectious diseases, perhaps even all of them. In addition, macrophages are the most common cells populating the atherosclerotic plaque (24). These cells originate from circulating monocytes or developing directly inside the plaque (25). Basic correlations between immunity, cancer and atherosclerosis are shown in Figure 1.
Trained immunity
Natural immunity, sometimes called trained immunity, plays a major role in switching from acute to chronic inflammation. For decades, the general dogma stated that natural immunity has no memory, which is characteristic for adaptive immunity only. However, numerous studies have clearly demonstrated that natural immunity is able to adapt. It also reveals that a contact with oxidized lipoprotein particle or with molecular patterns (PAMP) results in epigenetic modifications and program changes of monocytes and NK cells. These changes subsequently result in higher production of some cytokines and higher activity of anti-infection immunity against possible re-infection (26-28).
Adaptive immunity
Mechanisms of specific immune response, such as T and B lymphocyte cooperation and hierarchical secretion of cytokines and other humoral factors, are later involved in the formation of atherosclerotic plaque as well. Contrary to the situation in cancer immunity, these vectors are not directed against its own cells, but mostly against PAMP signals from modified lipids (29). In addition, no lymphatic structures, which are analogs of the secondary lymphatic tissues (e.g., ATLO), occur during cancer development. ATLO formation is well established during atherosclerosis (30,31).
Conclusions
This short study offers readers some new hypotheses on action mechanisms of immune factors involved in the development of two currently high-risk diseases: cancer and cardiovascular diseases. To be precise, these mechanisms involve the development of immunoediting during oncogenesis and actions of trained natural immunity and developing of characteristic tertiary lymphoid structures during atherogenesis. Current opinions believe that arteries represent tertiary lymphatic organs with variable cellular complexity, producing several immunocompetent factors. In these functions, ATLO are similar to secondary lymphoid tissues (23). Contrary to primary and secondary lymphoid organs and tissues which develop during embryogenesis as primary anlage, ATLO develop de novo postembryonically during chronic non-infectious inflammations. ATLO development can be divided into three distinct phases. In the first phase, ATLO arises in parallel with formation of atheroma without structured T and B zones, despite the fact that T and B chemoattractants are already produced. Next, a differentiation of T and B zones appears, accompanied by neogenesis of lymphatic venules associating with arterial wall. The final phase of ATLO is characterized by development of highly differentiated T and B cell follicles with active germinal centers, including dendrocytes and plasmacytes, and neogenesis of blood vessels.
Current information regarding the role of nutrition in the development and progress of these serious diseases, is rare and often even contradictory. It is important to keep in mind that nutrition directly affects the immune system and not the disease. Nutrition moves from the gut directly into the connected gut-associated lymphoid tissue (GALT), the largest immune organ. Immunocytes and humoral factors, which subsequently move to either cancer tissue or atherogenic plaque, are formed in the GALT. However, we do need to remember that nutrition affects microbiome. It seems it is highly important to focus on these aspects of nutrition. Nutrition may soon not only be the primary factor of prevention, but a direct therapeutic factor of numerous diseases, including cancer and atherosclerosis.
Acknowledgements
Funding: The authors wish to thank the grant RVO 61388971 for financial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ehrlich P. Address in Pathology, ON CHEMIOTHERAPY: Delivered before the Seventeenth International Congress of Medicine. Br Med J 1913;2:353-9. [Crossref] [PubMed]
- Ehrlich P. The partial function of cells. (Nobel Prize address given on 11 December 1908 at Stockholm). Int Arch Allergy Appl Immunol 1954;5:67-86. [Crossref] [PubMed]
- Ehrlich P. Über den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd 1909;5:273-90.
- Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J 1957;1:841-7. [Crossref] [PubMed]
- Thomas L. Discussion of Cellular and humoral aspects of the hypersensitive states. In: Lawrence HS. editor. Cellular and Humoral Aspects of the Hypersensitive States: A Symposium held at the New York Academy of Medicine. New York: Hoeber, 1959:529-32.
- Old LJ, Boyse EA. Immunology of Experimental Tumors. Annu Rev Med 1964;15:167-86. [Crossref] [PubMed]
- Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science 1974;183:534-6. [Crossref] [PubMed]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res 1975;22:261-422. [Crossref] [PubMed]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994;12:991-1045. [Crossref] [PubMed]
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol 2003;21:807-39. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002;2:301-10. [Crossref] [PubMed]
- Dighe AS, Richards E, Old LJ, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1994;1:447-56. [Crossref] [PubMed]
- Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-71. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007;121:1-14. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. [Crossref] [PubMed]
- Garrido F, Romero I, Aptsiauri N, et al. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer 2016;138:271-80. [Crossref] [PubMed]
- Borrego F, Ulbrecht M, Weiss EH, et al. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med 1998;187:813-8. [Crossref] [PubMed]
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591-6. [Crossref] [PubMed]
- Alwan A, Armstrong T, Bettcher D, et al. Global status report on noncommunicable diseases, World Health Organization, 2011:1-162.
- Yin C, Mohanta SK, Srikakulapu P, et al. Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity Front Immunol 2016;7:387. [Crossref] [PubMed]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341-55. [Crossref] [PubMed]
- Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166-72. [Crossref] [PubMed]
- Bekkering S, Joosten LA, van der Meer JW, et al. Trained innate immunity and atherosclerosis. Curr Opin Lipidol 2013;24:487-92. [Crossref] [PubMed]
- Bekkering S, Quintin J, Joosten LA, et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 2014;34:1731-8. [Crossref] [PubMed]
- Stevens WB, Netea MG, Kater AP, et al. 'Trained immunity': consequences for lymphoid malignancies. Haematologica 2016;101:1460-8. [Crossref] [PubMed]
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357-61. [Crossref] [PubMed]
- Akhavanpoor M, Gleissner CA, Akhavanpoor H, et al. Adventitial tertiary lymphoid organ classification in human atherosclerosis. Cardiovasc Pathol 2018;32:8-14. [Crossref] [PubMed]
- Sima P, Vannucci L, Vetvicka V. Atherosclerosis as autoimmune disease. Ann Transl Med 2018;6:116. [Crossref] [PubMed]