A case of pseudomembranous tracheitis caused by Mycoplasma pneumoniae in an immunocompetent patient
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is a cause of atypical pneumonia worldwide and occurs with a wide spectrum of symptoms (1). Radiologic findings of M. pneumoniae pneumonia are also diverse and nonspecific, although characteristic computed tomography (CT) features have been described (2,3).
Pseudomembranous tracheitis (PMT) is a rare condition characterized by pseudomembrane formation that involves the tracheobronchial tree. PMT caused by fungal infections, such as those caused by aspergillus fumigatus, has mostly been reported in immunocompromised hosts, such as those who underwent chemotherapy or transplantation (4,5). However, PMT can also be caused by bacterial species or in immunocompetent patients although a very rare basis (6-14). To our knowledge, PMT caused by M. pneumoniae has not yet been reported in immunocompetent adults. Here, we present a case of M. pneumoniae infection that caused PMT and bilateral diffuse bronchiolitis in a previously healthy adult.
Case presentation
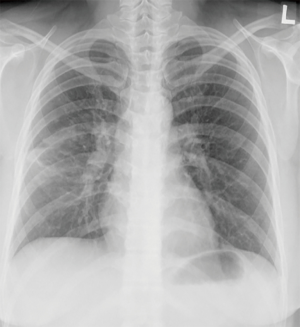
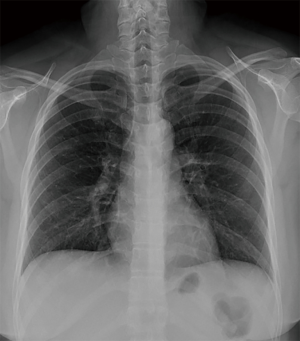
A 29-year-old woman with no history of underlying illness was presented as a transfer from an outside local medical center because of persistent coughing and chest radiographic deterioration (despite treatment with antibiotics) for suspected community acquired pneumonia. The patient was initially admitted because of dry cough, sore throat, and fever that lasted for 5 days. Her initial chest radiograph showed a slight peribronchial infiltration of the right lung (Figure 1), and she received intravenous ceftriaxone and clarithromycin treatment. After 7 days of treatment, cough and dyspnea deteriorated along with the worsening of the radiological finding; thus, she was transferred to our institution for further evaluation and management.
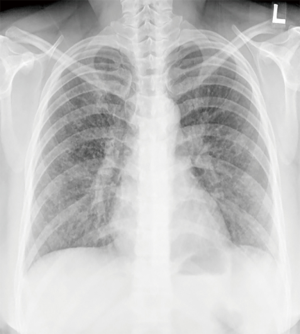
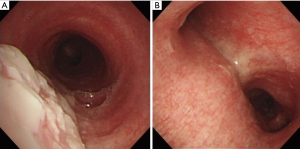
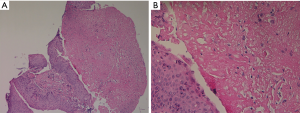
On admission, her vital signs were as follows: blood pressure, 156/87 mmHg; pulse rate, 72 beats/minute; respiratory rate, 25 breaths/minute; and body temperature, 37.5 °C. Oxygen saturation from pulse oximetry was 95% with 3 L/min of oxygen via nasal cannula. On auscultation, coarse breathing sounds were noted in both the lung fields. Laboratory test results were as follows: white blood cell count, 14,840 cells/mm3 (neutrophils, 84.7%); hemoglobin level, 12.4 g/dL; platelet count, 393,000 cells/mm3; lactic acid level, 2.6 mmol/L; and C-reactive protein level, 3.02 mg/dL. Procalcitonin level was 0.06 ng/mL (0–0.1 ng/mL). Other laboratory findings, including electrolyte and creatinine levels and liver function tests, were normal. No virus was identified from throat swab specimens using multiplex polymerase chain reaction (PCR) assay, which detects 16 respiratory viruses (Allplex Respiratory Panel; Seegene Biotechnology Inc., Korea). A urine antigen analysis for Streptococcus pneumoniae and Legionella pneumophila tested negative for both the organisms. A chest radiograph showed an increased peribronchial infiltration in both whole lung fields compared to the initial one (Figure 2). Chest CT showed diffusely scattered centrilobular micronodules and tree-in-bud opacity involving both the lungs (Figure 3). Also, asymmetrical wall thickening with contrast enhancement was observed in membranous portion of the upper part of trachea. Given the poor response to previous antimicrobial therapy, antibiotics were switched to intravenous piperacillin/tazobactam and moxifloxacin. In addition, bronchoscopy was performed to determine the presence of atypical pathogen or noninfectious processes. The vocal cords were not swollen and inflamed. Mucosal ulceration and white exudative plaques, partially covering the membranous portion of the upper and middle trachea, were noted, whereas the distal trachea and all the bronchi were unremarkable except for the mucosal hyperemia (Figure 4). Bronchial aspirate from the lesion were examined using GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, USA) and AmpliSens Mycoplasma pneumoniae/Chlamydia pneumoniae-FRT PCR kit (InterLabService Ltd., Russia), and the results were positive for M. pneumoniae. However, microbial staining and culture examination for common bacteria, fungi, and Mycobacterium tuberculosis were all negative in those specimens. A biopsy of the pseudomembrane revealed inflammatory exudate with necrotic debris and squamous metaplasia (Figure 5). Necrotic tracheitis was diagnosed. Subsequently, tests for IgM and IgG antibodies against M. pneumoniae were positive [IgM >27 index (<10 index); IgG 86.3 AU/mL (<10 AU/mL)]. Testing for cold agglutinin also revealed positive findings [1:32 (≤1:4)]. Serological tests regarding autoimmune diseases and other atypical pathogens, including human immunodeficiency virus (HIV), were negative. Some relevant laboratory findings are summarized in Table 1.

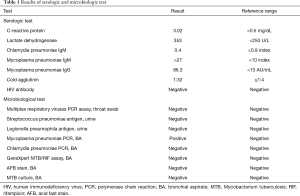
Full table
While continuing the antibiotic therapy, 20 mg methylprednisolone was administered daily for relieving severe cough that did not respond to previous cough medicines. On the sixth day of hospitalization, the patient was discharged on oral moxifloxacin, with remarkable improvement in cough and radiologic findings on chest X-ray (Figure 6). She was found to have completely recovered without residual symptoms in the outpatient clinic after discharge although improvement of tracheal lesion was not identified by follow-up bronchoscopy because she declined it.
Discussion
M. pneumoniae infection causes diseases of varied severity, ranging from minor respiratory illness to severe atypical pneumonia (1). Our patient who presented with severe cough, worsening of dyspnea, and deterioration of radiologic findings was found to have M. pneumoniae infection, unusually causing PMT associated with bilateral diffuse bronchiolitis. Here, we presented a rare case of an immunocompetent adult with PMT and bilateral diffuse bronchiolitis caused due to M. pneumoniae.
PMT is characterized by presence of extensive inflammation and invasion of the tracheobronchial trees with formation of a pseudomembrane composed of fibrin, leukocytes, and possibly organism overlying a damaged airway mucous membrane (4). It has been proposed that diminished function of neutrophils and macrophages is implicated in the development of PMT (15). Cases with PMT have been reported in association with fungal infections caused by Aspergillus, Candida, and Rhizopus. Other rare pathogens include Pseudomonas, Corynebacterium, Bacillus, Staphylococcus, Moraxella, and Chlamydia species (4). Noninfectious causes are inflammatory bowel disease, endotracheal intubation, and post-transplantation (4). Most of them are usually developed in immunocompromised hosts and have a high morbidity and mortality. Therefore, patients suspected of having PMT need a thorough diagnostic evaluation and prompt and proper treatment for the causative organism.
Our patient did not have any of the above antecedent factors, and there was no evidence of any infection with a specific pathogen known to cause PMT. M. pneumoniae infection as the cause of her condition was supported by (I) radiological finding of centrilobular nodules compatible to M. pneumoniae bronchiolitis (2,3), (II) a positive PCR result for M. pneumoniae from the lesion, and (III) elevated serum antibody titer for M. pneumoniae. Thus, although the pathogenesis was unclear, tracheal pseudomembranous lesion was most likely caused by the M. pneumoniae infection. Extremely severe coughing, as seen in this case, can probably be attributed to PMT, given that the sensory nerves responsible for cough are predominantly in the upper airway, including the trachea, especially the membranous portion (16). In fact, bronchoscopy is not generally performed for patients with M. pneumoniae respiratory infection. Thus, the frequency of such PMT might not be so rare. Therefore, PMT can be considered in a patient with complaints of intractable cough without any response to the usual treatment for M. pneumoniae infection and having tracheal wall thickening in chest CT. Bronchoscopy should be performed in such circumstances.
Although data regarding the CT findings of M. pneumoniae infection are limited, reported findings include centrilobular nodular and tree-in-bud opacities in a patchy distribution, lobular or segmental ground-glass opacities or consolidation, and thickening of the bronchovascular bundle (2,3). In terms of the distribution of nodules on a CT scan, a patchy distribution favors infectious bronchiolitis, while diffuse distribution is usually observed in noninfectious causes, including hypersensitivity pneumonitis (17). In this aspect, diffuse distribution of centrilobular nodules throughout both the lungs in our case hindered the consideration of M. pneumoniae infection in the first impression. However, bronchoscopically identified tracheal lesions provided a relevant answer for these atypical, diffuse distributions of centrilobular nodules observed in our case. Tracheal mucosal lesions with necrotic materials caused by M. pneumoniae may lead to endobronchial spreading throughout the bilateral bronchial tree, resulting in a form of diffuse bronchiolitis during the clinical course.
Our patient did not show prompt improvement in response to clarithromycin administration received for 1 week before referral to our hospital. Macrolide-resistant M. pneumoniae is an emerging problem (1). Although the impact of macrolide resistance on the outcomes of respiratory infection is unclear, the clinical course of patients with macrolide-resistant M. pneumoniae infection tends to be prolonged. However, the severity of the clinical illness may correlate with an individual’s immune response to M. pneumoniae regardless of antimicrobial resistance (18). In this context, a prolonged clinical course observed in our patient may not be associated with macrolide failure; it may rather be associated with a complicated PMT or exacerbated immune response although in-vitro macrolide susceptibility data were not obtained in this case.
Adjunctive corticosteroid therapy was administered to the patient for 5 days, following which her persistent and severe coughing with no response to prior antibiotics and cough medicines rapidly improved. Although the benefits of adjunctive steroids are controversial in patients with M. pneumoniae pneumonia (19), administering steroids in addition to effective antibiotics may be a good option for rapidly relieving persistent and severe coughing caused by PMT. In addition, clinical outcomes of immunocompetent patients with PMT caused by M. pneumoniae seem favorable compared to grave outcomes in cases with immunocompromised conditions or fungal infection. Several reported cases of PMT developing in immunocompetent patients have been outlined in Table 2 (6-14). Most cases had favorable outcomes like our case.
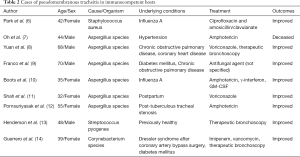
Full table
In conclusion, we presented the first case of PMT associated with bilateral diffuse bronchiolitis caused by M. pneumoniae infection that showed a complete symptomatic and radiographic recovery after administration of moxifloxacin and adjunctive corticosteroids. M. pneumoniae should be considered as a causative pathogen in immunocompetent adult patients with bilateral diffuse bronchiolitis and PMT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report.
References
- Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 2008;32:956-73. [Crossref] [PubMed]
- Reittner P, Muller NL, Heyneman L, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol 2000;174:37-41. [Crossref] [PubMed]
- Miyashita N, Sugiu T, Kawai Y, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging 2009;9:7. [Crossref] [PubMed]
- Malhotra P, Singh K, Gill P, et al. Pseudomembranous tracheitis caused by Aspergillus fumigatus in the setting of high grade T-cell lymphoma. Respir Med Case Rep 2017;21:42-5. [Crossref] [PubMed]
- Williams KE, Parish JM, Lyng PJ, et al. Pseudomembranous tracheobronchitis caused by Rhizopus sp. After allogeneic stem cell transplantation. J Bronchology Interv Pulmonol 2014;21:166-9. [Crossref] [PubMed]
- Park SS, Kim SH, Kim M, et al. A Case of Severe Pseudomembranous Tracheobronchitis Complicated by Co-infection of Influenza A (H1N1) and Staphylococcus aureus in an Immunocompetent Patient. Tuberc Respir Dis (Seoul) 2015;78:366-70. [Crossref] [PubMed]
- Oh HJ, Kim HR, Hwang KE, et al. Case of pseudomembranous necrotizing tracheobronchial aspergillosis in an immunocompetent host. Korean J Intern Med 2006;21:279-82. [Crossref] [PubMed]
- Yuan ML, Yang G, Hu HL, et al. A case of Aspergillus tracheobronchitis in a patient with chronic obstructive pulmonary disease. Indian J Pathol Microbiol 2017;60:285-7. [Crossref] [PubMed]
- Franco J, Muñoz C, Vila B, et al. Pseudomembranous invasive tracheobronchial aspergillosis. Thorax 2004;59:452. [Crossref] [PubMed]
- Boots RJ, Paterson DL, Allworth AM, et al. Successful treatment of post-influenza pseudomembranous necrotising bronchial aspergillosis with liposomal amphotericin, inhaled amphotericin B, gamma interferon and GM-CSF. Thorax 1999;54:1047-9. [Crossref] [PubMed]
- Shah M, Singhal P. Rare Case: Invasive Pseudomembranous Aspergillus Tracheobronchitis in a Postpartum Patient Presenting With Stridor. J Bronchology Interv Pulmonol 2015;22:248-50. [Crossref] [PubMed]
- Pornsuriyasak P, Murgu S, Colt H. Pseudomembranous aspergillus tracheobronchitis superimposed on post-tuberculosis tracheal stenosis. Respirology 2009;14:144-7. [Crossref] [PubMed]
- Henderson MH, Spradley CD, DeKeratry DR. Pseudomembranous tracheobronchitis due to streptococcus pyogenes: a case report. Chest 2009;136:abstr 7S-e.
- Guerrero J, Mallur P, Folch E, et al. Necrotizing tracheitis secondary to corynebacterium species presenting with central airway obstruction. Respir Care 2014;59:e5-8. [Crossref] [PubMed]
- Mehrad B, Paciocco G, Martinez FJ, et al. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest 2001;119:169-75. [Crossref] [PubMed]
- Fuller RW, Jackson DM. Physiology and treatment of cough. Thorax 1990;45:425-30. [Crossref] [PubMed]
- Gruden JF, Webb WR, Naidich DP, et al. Multinodular disease: anatomic localization at thin-section CT--multireader evaluation of a simple algorithm. Radiology 1999;210:711-20. [Crossref] [PubMed]
- Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol 2007;56:1625-9. [Crossref] [PubMed]
- Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: A Potentially Severe Infection. J Clin Med Res 2018;10:535-44. [Crossref] [PubMed]