
A paradigm shift in biomarker guided oncology drug development
Introduction
For decades, oncology drugs have been developed and approved for conventionally-defined cancer indications based on tumor histological findings related to a specific anatomic location and clinical data from all-comers clinical trials. However, with the development of trastuzumab (Herceptin, Roche/Genentech) for metastatic HER2 positive breast cancer, drug development entered a new era where predictive biomarkers played a decisive role in the drug development process. According to the FDA-NIH Biomarker Working Group a predictive biomarker is used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent (1). For trastuzumab, the hypothesis was that the drug was effective in patients who had either an amplification of the HER2 gene or an overexpression of the HER2 protein (2,3). When Genentech did the clinical development of trastuzumab they used an HER2 immunohistochemical (IHC) assay to select the likely responding patients and thereby introduced the enrichment clinical trial design. Their hypothesis about the link between the HER2 expression and the efficacy of trastuzumab proved to be right (4). In September 1998, trastuzumab and the IHC assay for HER2 expression (HercepTest, Dako) simultaneously obtained approval by the US FDA and became the first regulator-approved drug-diagnostic combination to be used in the clinic (5).
Since the turn of the century, more than 30 targeted anti-cancer drugs have been approved together with a companion diagnostic assay (CDx) and have significantly improved the treatment outcome for a number of patients (6,7). Although these drugs are guided by a CDx assay they have still been developed based on a conventionally-defined tumor classification and not on a site agnostic approach, as known from the clinical “basket” trial design (8). However, by the end of November 2018, the US FDA granted accelerated approval to the tropomyosin receptor kinase (TRK) inhibitor larotrectinib (Vitrakvi, Loxo Oncology and Bayer) for adult and pediatric patients with solid tumors that have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion (9,10). What makes this approval exceptional is that for the first time a drug has been approved solely on its efficacy related to the presence of specific molecular aberrations and not on a conventional cancer indication based on tumor histology and anatomical location. The clinical data that led to the approval of larotrectinib comprised of 12 different conventional cancer indications; however, with one common denominator that their tumors harbored a NTRK gene fusion (10). It is not the first time the US FDA have granted an approval based on the presence of specific molecular aberrations. In May 2017, the immune checkpoint inhibitor pembrolizumab (Keytruda, Merck Sharp and Dohme) obtained approval of a new indication for treatment of patients with microsatellite instability-high (MSI-H) or mis-match-repair-deficient (dMMR) solid tumors (11).
Drug-diagnostic co-development
Ideally, any drug development project should rely on an in-depth molecular understanding of the pathophysiology and the drug mechanism of action. Especially within oncology, the advances in molecular diagnostics have given us insights into the disease mechanisms in recent years. This new insight has enabled us to practice a more rational drug development process, where a CDx assay is developed in parallel to the drug using the drug-diagnostic co-development model (12). In this model, the assumption is that specific molecular characteristics need to be present in order for the drug to be effective. This means that molecular testing becomes an important part of the patient selection process for the clinical development of the drug. However, before a CDx assay can be used in a pivotal clinical trial it must undergo an intensive analytical validation program. During this program, the assay is tested with regard to variables such as sensitivity, specificity, robustness, repeatability, and reproducibility. The analytical validation is important in order to avoid false-positive and false-negative test results, which could have serious consequences for the patient selection process and hence the whole drug development program (12,13).
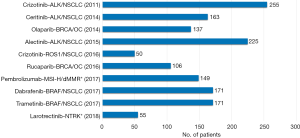
In drug development programs where the enrichment trial design has been used, the efficacy of several targeted anti-cancer drugs has been based on surprisingly small patient populations. The main reason for this is that testing with the CDx assay screens out a large part of the non-responding patients. Figure 1 shows examples of targeted anti-cancer drugs that have obtained US FDA approval together with their CDx assay based on efficacy data from relatively few patients in single-arm enrichment studies (14). However, for most of the drugs mentioned in Figure 1 it is important to note that their market authorizations were granted based on accelerated approvals, which means that they have to meet a number of additional requirements during the post approval phase (13).
Over the last few years, we have experienced an increasing number of “basket” trials being initiated where the investigational drug is studied for more than one conventional cancer indication simultaneously. In these trials, the patients are enrolled based on a common specific molecular aberration rather than on tumor histology and anatomic location (8,11,15). For both pembrolizumab in relation to the MSI-H/dMMR indication and for larotrectinib in relation to patients with NTRK gene fusion, a similar approach has been used. However, due to the low frequency of the specific molecular aberrations, patient data from different trials was pooled in order to document the efficacy of the drugs across the different cancer indications. For pembrolizumab data from five different trials was pooled and for larotrectinib the number was three (10,16).
Despite the principle of the “basket” trials is appealing there seem to be challenges and so far, only a few clinical trials with successful outcome were reported, such as the recent trials with pembrolizumab and larotrectinib. In a Roche funded “basket” trial with vemurafenib (Zelboraf, Roche/Genentech), in BRAF V600 mutation-positive patients, only a few of the conventionally-defined cancer indications responded to the treatment despite being mutation-positive (17). The cohort of patients with non-small cell lung cancer (NSCLC) showed an objective response rate (ORR) of 42% (95% CI, 20–67%). A similar ORR, of 43% (95% CI, 18–71%) was obtained for the cohort of patients with Erdheim-Chester disease or Langerhans cell histiocytosis. For the remaining conventional cancer indications, such as anaplastic thyroid cancer, cholangiocarcinoma, salivary-duct cancer, and ovarian cancer only anecdotal responses were reported. Furthermore, another recent published “basket” trial with neratinib (Nerlynx, Puma Biotechnology), a pan-HER kinase inhibitor, in patients harboring HER2 or HER3 mutation showed mixed results (18). In the cohort of patients with HER2 mutation response to neratinib was found in breast cancer, biliary tract cancer, cervical cancer, and lung cancer but not in bladder cancer, colorectal cancer (CRC), endometrial cancer, gastroesophageal cancer, and ovarian cancer. Likewise, more than 20 years of clinical research with trastuzumab in HER2 positive cancer patients has only led to the approval of two indications; breast cancer and gastroesophageal cancer. Based on the experience so far, we can conclude that tumor site and histology might play a role in relation to the efficacy of targeted anti-cancer drugs. It seems that not every oncogenic driver found in the tumor is targetable regardless of origin, and a complex interaction between the molecular aberrations and tumor histology might exist.
Pembrolizumab and MSI-H or dMMR
Pembrolizumab belongs to a new class of anti-cancer drugs called immune checkpoint inhibitors. These compounds are antibodies that blocks the interaction between programmed death ligand 1 (PD-L1) and its receptor PD-1, whereby the host immunity is restored resulting in enhanced T-cell response and increased antitumor activity (19). In September 2014, pembrolizumab was regulatory approved for the first time by the US FDA for treatment of metastatic melanoma, and has subsequently been approved for a number of different cancer indications such as NSCLC, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma and more (16).
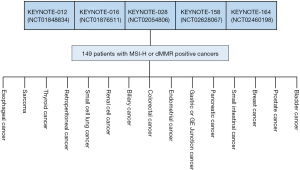
In May 2017, pembrolizumab was approved for the treatment of MSI-H or dMMR solid tumors. This approval was based on data pooled from five individual multicentre, multi-cohort, single-arm enrichment trials (11,16,20). A total of 149 patients were enrolled across these five clinical trials, of which 98% had a metastatic disease. Figure 2 gives an overview of how the data was pooled and of the individually-defined conventional cancer indications included in the different trials. The primary efficacy endpoints were anti-tumor activity measured as ORR assessed blinded by independent central radiologists according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and duration of response (DOR). More than half of the patients had a conventional diagnosis of CRC and the remaining group (39.6%) consisted of 14 different conventional diagnoses with endometrial cancer, biliary cancer, and gastroesophageal cancer being the most frequent. Of the 149 cancer patients, 47 had dMMR identified by IHC, 60 had MSI-H assessed by PCR, and both tests were used on 42. The ORR for all patients were 39.6% (95% CI, 32–48%) with more than 78% of the patients responding after 6 months and a corresponding DOR range of 1.6+ to 22.7+ months. The + after the number of months denotes an ongoing response. Comparing the CRC group with the non-CRC group, the latter group seems to response slightly better to the treatment with pembrolizumab. For the 59 patients in the non-CRC group ORR was 45.8% (95% CI, 33–59%) and for the CRC group the corresponding figures were 35.6% (95% CI, 26–46%) (16). A couple of other recent studies with pembrolizumab in the same type of patients have shown similar outcome which adds to the evidence that these tumors are sensitive to treatment with this immune checkpoint inhibitor regardless of histology and anatomical site (21,22).
One shortcoming in relation to the approval of pembrolizumab for this new site agnostic indication was the lack of a concomitant regulator-approved CDx assay. The identification of patients with MSH-D or dMMR tumors was performed by local laboratory-developed tests, which add to an increased test-to-test variability that likely results in a more heterogenous patient population across the clinical test sites. Especially for this type of indication, the availability of a fully analytical validated CDx assay is of paramount importance at the time of initiation of the pivotal clinical trials, since the assay defines the patient population. The US FDA explained this unusual exception by the fact of highly unmet medical needs, high response rate, and the known safety profile of pembrolizumab (11). However, in relation to the post approval requirements, Merck Sharp and Dohme, the producers of pembrolizumab, committed themselves to develop a fully validated assay. Furthermore, in October 2018, the US FDA issued a guideline on “Developing Targeted Therapies in Low-Frequency Molecular Subsets of a Disease” and here, it is underlined that it is essential for these types of drugs that a US FDA approved CDx assay is available at the time of drug approval in order to identify patients in the clinical setting. However, the guideline also states that the US FDA may grant exceptions when the drug is intended for treatment of a serious or life-threatening condition for which no satisfactory alternative treatment exists (23).
Larotrectinib and NTRK fusions
Larotrectinib is an inhibitor of the tropomyosin receptor kinases TRKA, TRKB, and TRKC, which are encoded by the genes NTRK1, NTRK2, and NTRK3. Chromosomal rearrangements involving in-frame fusions of these genes with various partners can result in constitutively-activated chimeric TRK fusion proteins that can act as an oncogenic driver, promoting cell proliferation and tumor cell survival (10,24). In vitro and in vivo models as well as early clinical evidence have suggested that these TRK gene fusions lead to oncogenic addiction regardless of tissue or origin, and it has been estimated that up to 1% of all solid tumors may be implicated, and comprise both adult and pediatric patients (25,26).
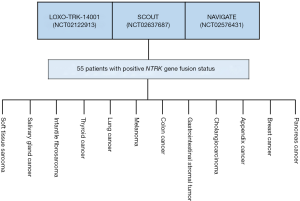
The approval of larotrectinib was granted for treatment of adult and pediatric patients with solid tumors that have a NTRK gene fusion. In addition, patients who might be candidates for treatment must be without known acquired resistance mutation and have either metastatic disease or be in a position where a surgical resection is likely to result in severe morbidity. Furthermore, the patients must have no satisfactory alternative treatments or the tumor has progressed following the preceding treatment (10). The approval of larotrectinib was, as for pembrolizumab, based on pooled data from several independent clinical trials. The efficacy documentation comprised of data from a total of 55 adult and pediatric patients enrolled in three phase 1 and 2 multicenter, open-label, single-arm enrichment trials. Identification of patients positive for NTRK gene fusion was performed prospectively by local laboratory-developed tests, either by next generation sequencing (N=50) or fluorescence in situ hybridization (N=5). As discussed in relation to pembrolizumab, the use of different local laboratory-developed tests must be regarded as a major shortcoming due to the extra variability they add to the patient selection process as well as to the overall definition of the patient population. Figure 3 gives an overview of how the data was pooled from the three clinical trials that made up the efficacy documentation for larotrectinib and the individually-defined conventional cancer indications. The most common cancer indications were salivary gland cancer (22%), soft tissue sarcoma (20%), infantile fibrosarcoma (13%), and thyroid cancer (9%). The age of the patients ranges from 4 months to 76 years, and 82% of the patients were metastatic and 18% had locally advanced, unresectable disease. Ninety-two percent of patients had received prior treatment for their disease, including surgery, radiotherapy, or systemic therapy (10).
The primary efficacy endpoints in the three clinical trial were anti-tumor activity measured as ORR and DOR, as determined by a blinded independent review committee according to RECIST v1.1 (10). The ORR for all patients were 75% (95% CI, 61–85%) with 73% of the patients responding after 6 months and a corresponding DOR range of 1.6+ to 33.2+ months. The group of patients with soft tissue sarcoma (N=12) achieved an ORR of 91% (95% CI, 59–100%) and a DOR ranging from 3.6 to 33.2+ months. For the group of patients with infantile fibrosarcoma (N=7) and thyroid cancer (N=5) the ORR was 100%. The most frequent fusion partners were ETV6-NTRK3, which were found in 45% of all patients (10). For some of the nonresponding patients concerns have been raised with regard to some of the local laboratory-developed assays used, which might have come up with a false positive NTRK gene fusion test result (25).
As with most anti-cancer drugs, acquired resistance was observed following treatment with larotrectinib. This was also the situation for 10 patients in the three different trials, who experienced disease progression during treatment after they had achieved an objective response or stable disease for at least 6 months. Sequencing tumor and plasma samples from these patients revealed that mutations altered the kinase domain of the TRK, which explained most of the progression events. The detection of such mutations might soon be relevant as the next generation of TRK inhibitors are under development, which is especially designed to address acquired kinase domain mutations. The compound LOXO-195 is currently being evaluated in early clinical development and has already demonstrated clinical activity in a few patients (25-27).
The data used for documenting efficacy and safety of larotrectinib in relation to the US FDA regulatory approval has recently been published in New England Journal of Medicine and Lancet Oncology, respectively (25,26).
Summary and conclusions
Over the past couple of decades, biomarker driven enrichment clinical trials have proven to be an important tool in clinical drug development, especially for targeted anti-cancer drugs. By the end of 2018, more than 30 drugs have been developed in conjunction with a biomarker test and have a regulator-approved CDx assay linked to their use (6). However, these drugs have mainly been developed for a single conventionally-defined cancer indication for patients who harbor a specific oncogenic driver, such as HER2 positive breast cancer and EGFR mutation positive NSCLC. Compared to these drugs, larotrectinib stands out as the first drug solely to be developed based on the presence of specific molecular aberrations and not on a cancer indication related to a specific tumor histology and anatomical location. Across 12 different conventionally-defined NTRK gene fusion positive cancer indications larotrectinib showed an ORR as high as 75%, and for some of the tumor types, such as infantile fibrosarcoma and thyroid cancer, the ORR of 100% was achieved (10).
In May 2017, the first step was taken that paved the way for a paradigm shift in biomarker guided drug development, when pembrolizumab was approved for treatment of patients with MSI-H or dMMR solid tumors (11,16,20). However, by that time pembrolizumab had been on the market for more than three years and had been approved for several conventionally-defined cancer indications. So, despite the MSI-H/dMMR indication was groundbreaking, it was not this indication that formed the basis for the overall approval of the drug, which is the situation for larotrectinib. Today, pembrolizumab is approved for 11 different cancer indication, including treatment of patients with MSI-H or dMMR solid tumors (16).
With the recent positive results with pembrolizumab and larotrectinib, clinical development based on a tumor and site agnostic approach seems to be an option worth to pursue. However, results from the few “basket” trials published, so far, seem to indicate that oncogenic drivers cannot always be used universally to select patients for a specific targeted anti-cancer treatment (17,18). There is still a lot to learn when it comes to the pharmacological action of anti-cancer drugs and it seems that tumor histology and site in conjunction with the oncogenic drivers may play a role for the mechanism of action.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jan Trøst Jørgensen has worked as a consultant for Agilent Technologies, Euro Diagnostica, Azanta and Oncology Venture and has given lectures at meetings sponsored by AstraZeneca, Merck Sharp & Dohme, and Roche.
References
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); 2016. FDA-NIH Biomarker Working Group. 2016 Jan 28. Updated 2017 Sep 25. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338449/. Accessed February 21, 2019.
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Jørgensen JT, Winther H. The development of the HercepTest – from bench to bedside. In: Jørgensen JT, Winther H. editors. Molecular Diagnostics: The Key Driver in Personalized Cancer Medicine. Pan Stanford, 2010:43-60.
- US FDA. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Updated: 12/06/2018. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm. Accessed December 10, 2018.
- Jørgensen JT. Clinical application of companion diagnostics. Trends Mol Med 2015;21:405-7. [Crossref] [PubMed]
- André F.. Developing Anticancer Drugs in Orphan Molecular Entities - A Paradigm under Construction. N Engl J Med 2018;378:763-5. [Crossref] [PubMed]
- US FDA. FDA approves larotrectinib for solid tumors with NTRK gene fusions. November 27, 2018. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626720.htm. Accessed November 28, 2018.
- US FDA. Prescribing Information for Vitrakvi (larotrectinib). Revised: 11/2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211710s000lbl.pdf. Accessed November 30, 2018.
- Lemery S, Keegan P, Pazdur R.. First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med 2017;377:1409-12. [Crossref] [PubMed]
- Jørgensen JT, Hersom M. Companion diagnostics-a tool to improve pharmacotherapy. Ann Transl Med 2016;4:482. [Crossref] [PubMed]
- Jørgensen JT, Hersom M. Clinical and Regulatory Aspects of Companion Diagnostic Development in Oncology. Clin Pharmacol Ther 2018;103:999-1008. [Crossref] [PubMed]
- US FDA. Drugs@FDA: FDA Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed December 2, 2018.
- Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med 2017;377:62-70. [Crossref] [PubMed]
- US FDA. Prescribing information for Keytruda (Pembrolizumab). December 19, 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s045lbl.pdf. Accessed December 21, 2018.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554:189-94. [Crossref] [PubMed]
- Hersom M, Jørgensen JT. Companion and Complementary Diagnostics-Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Ther Drug Monit 2018;40:9-16. [PubMed]
- Jørgensen JT. When biomarkers define a drug indication. Expert Rev Mol Diagn 2018;18:315-7. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- US FDA. Developing Targeted Therapies in Low-Frequency Molecular Subsets of a Disease. Guidance for Industry. Clinical Pharmacology. October 2018. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM588884.pdf. Accessed December 3. 2018.
- Dolgin E.. Loxo TRK inhibitor data wows oncologists. Nat Biotechnol 2017;35:694-95. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 2018;19:705-14. [Crossref] [PubMed]
- Drilon A, Nagasubramanian R, Blake JF, et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov 2017;7:963-72. [Crossref] [PubMed]


