
VCAM-1+ macrophage subset as ‘educators’ in fetal liver for transition to definitive hematopoiesis
Hematopoietic ontogeny has been well studied and are described in the literature as waves of activity (1). Intuitively, it is expected that early hematopoiesis will begin in the yolk sac since the early embryo will need support from blood cells, commonly termed the first wave (2). Consistent with the need for blood cells during the first wave, this period produces nucleated erythrocytes to ensure sufficient oxygenation. The second wave begins to generate a broader population of primitive hematopoiesis that can produce myeloid and lymphoid cells. This begins to prepare the nascent hematopoietic stem cells (HSCs) to leave the embryonic niche of early development to the fetal liver. The HSCs that are permanent after birth and throughout the life of an individual are populated in the bone marrow during the third wave of embryonic hematopoiesis in the aorta gonad-mesonephros (AGM) region (3).
Several groups have reported on methods to develop HSCs from embryonic stem cells (ESCs) and induced pluripotent stem cells. The literature reported on human ESC-derived HSCs reconstituting immune deficient mice and generate functional mature immune cells such as monocytes and neutrophils (4-6). The ESC-generated HSCs however, do not behave as native HSCs. As examples, the ESC-derived HSCs cannot respond to stressors in vivo and failed to sustain serial passaging, which is the hallmark of pluripotency (7). Part of the issue of generating functional HSCs in vitro may be partly explained by the gap in the knowledge of how nascent HSCs are educated as they migrate to the bone marrow. Thus, when the HSCs are generated from ESCs and iPSCs, the ‘educational’ process required for effective function is missing. Additionally, the literature shows a gap in the molecular and detailed migratory path by which nascent HSCs migrate to the fetal liver and once in this organ, the region of retention. Also, it is unclear if the time spent in the fetal liver is a function hematopoietic activity in the bone marrow. Such questions are fundamental to bone marrow transplant, generation of HSCs from pluripotent stem cells and, the treatment of hematological dysfunction.
The development of endogenous HSCs uses a complex ‘educational’ process, beginning in the yolk sac during the first wave and later through the fetal liver (2). Despite studies to shown the homing processes of HSCs during development, the detailed cellular and molecular mechanisms remain unresolved. Answers to these questions were partly addressed by Li and colleagues (8). The authors used models of Zebrafish with a library of mutants linked to hematopoietic dysfunction. Their screening resulted in the identification of integrin alpha 4 mutant (itga4) in definitive hematopoietic defects. Interestingly, these defects were not due to changes in primitive hematopoiesis or vascular morphogenesis, indicating that the defects occur after the generation of nascent HSCs and at some points between the developing nascent HSCs and the path through the fetal liver prior to the last stage in bone marrow.
Since the itga4 mutants showed hematopoietic defects, the authors examined different regions for its expression. The expression of itga4 was noted in the region where nascent HSCs were generated in the AGM, and the fetal liver (vertebrates) or in the case of zebrafish, the surrogate organ is the caudal hematopoietic tissue (CHT). Since itga4 expression depended on transcription factors required for the emergence of nascent HSCs (runx1 and myb), it was deduced that itga4 must be important in the educational process of nascent HSCs in the fetal liver. Previous studies have reported on a role for VLA-4 integrin, with itga4 and itgb1 subunits (α- and β-subunits), in definitive hematopoiesis (9). This supported further investigation into the role if itga4 in the process of the nascent HSCs generating definitive hematopoiesis.
The authors began by asking if the itga4 mutants affected definitive hematopoiesis and such dysfunction did not occur because of problems to generate nascent HSCs. To address this, the investigators used a fluorescence reporter gene system under the control of the vascular growth factor receptor 1 that could identify the conversion of hemogenic endothelial cells within the AGM to nascent HSCs (runx1+/myb+) and then track the nascent HSCs to the fetal liver (vertebrates)/CHT (zebrafish). The studies using wild type and itga4 mutant zebrafish showed the time spent by HSCs in the fetal liver was ~8 fold more with wild type than the mutant. More importantly, the authors showed a function between the time nascent HSCs spent in the fetal liver and definitive hematopoiesis.
The wild type nascent HSCs found their niche within the dorsal region of the caudal venous plexus (CVP) of fetal liver (CHT in the zebrafish). The areas were not random and were therefore designated as hot spots. These hot spots were located at the confluence points of the venous capillary that are linked to the CVP where the velocity of the nascent HSCs showed a significant reduction and with diameter similar to the nascent HSCs. It was therefore proposed that the reduced velocity facilitated the nascent HSCs to be subjected to an ‘educational’ process by other nearby niche cells. The retention noted for wild-type zebrafish was not seen in itga4 mutants. Since the difference could be explained by morphological changes of the vascular system of the itga4 mutants, the authors examined this further. They noted no change in the vascular region within the fetal liver (CHT). This led them to propose that other components within the fetal liver could be involved in facilitating nascent HSC retention.
Since itga4 is a subunit for VLA-4 integrin, the authors focused on its ligand, vascular cell adhesion molecule-1 (VCAM-1), as the causative binding partner for itga4 to retain nascent HSCs in the fetal liver. Indeed, the authors noted a strong expression of VCAM-1 in the hot spot regions within the fetal liver and also noted that VCAM-1 was not present on endothelial cells, leading them to seek another niche cell. They eliminated the involvement of hematopoietic stromal cells and CXCL12-associated reticular cells as the candidate VCAM-1 expressing cells. Using their engineered zebrafish, the authors identified VCAM-1+ macrophages (MΦ) as those interacting with the HSCs. The VCAM-1+ MΦ was a MΦ subtype but these particular cells were shown to be fundamental in the educational process of HSCs in the fetal liver. This was based on loss of function studies through MΦ depletion. The investigators asked for the source of the VCAM1+ MΦs through timeline appearance of these cells in the fetal liver and compared with the time of the emerging nascent HSCs. They noted that the VCAM1+ MΦs appeared in the fetal liver before the emergence of nascent HSCs in the AGM, suggesting that the educational MΦs in the fetal liver must be derived from an early MΦ precursor. Thus, the findings strongly suggested that the VCAM1+ MΦs must be dispersed from a very primitive MΦ in the AGM to the fetal liver where they prepare the niche for incoming nascent HSCs.
Live imaging shows that all of the nascent HSCs could not interact with the VCAM1+ MΦs. The authors characterized the retention time and location into based on these parameters, categorized the type of retention into three groups. The subset that can remain the longest in the hot spots were likely to succeed in generating definitive hematopoiesis. Due to the ability of the VCAM1+ MΦs to direct the time spent in transit to the bone marrow (kidney marrow in the zebrafish), they were designated ‘usher’ cells.
The studies fell short from explaining what happened to the nascent HSCs that could not be retained in the fetal liver. It is possible that they could still form definitive hematopoiesis in the adult marrow and thymus since the itga4 mutation was limited to defects of definitive hematopoiesis in the fetal liver but not the thymus and marrow. Figure 1 broadly summarizes the overall findings of the publication by Li et al. (8). The diagram places the findings in the context of vertebrates. Shown are the emergence of nascent HSCs known to express runx1 and myb within the AGM. In parallel are the generation of primitive MΦs that generate the VCAM1+ MΦ that enters the fetal liver. There the MΦs interact with the nascent HSCs via VCAM1 (ligand) and VLA4 (receptor). The retention time of the nascent HSCs is proportional to the ability of these cells to develop into definitive hematopoiesis. The Figure questions if the role of itga4 in hematopoietic activity in bone marrow. Although this point was not stressed in the manuscript, the authors alluded to this, suggesting that there might be more cells and molecules to fully understand the ontogeny from AGM to bone marrow.
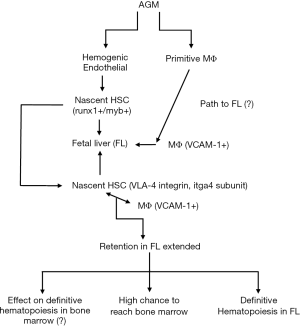
Overall, Li et al. built their research on other studies in the literature. However, their approach was novel as they systematically traced the step shown in Figure 1 using a zebrafish model. This allowed the authors to perform large scale library screening. The reduced the cost as one would expect from the use of knockout mice and at the same time, reduce killing of mice unless required. The findings can now form the basis to identify other molecules on the usher MΦs and to identify other niche cells. Interaction between the usher MΦ and nascent HSCs could not explain activity in the marrow and thymus. These areas remain unresolved issues that must be addressed as the method could be used to address the aging process in bone marrow and thymus.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ivanovs A, Rybtsov S, Ng ES, et al. Human haematopoietic stem cell development: from the embryo to the dish. Development 2017;144:2323-37. [Crossref] [PubMed]
- Frame JM, Fegan KH, Conway SJ, et al. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells 2016;34:431-44. [Crossref] [PubMed]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996;86:897-906. [Crossref] [PubMed]
- Yokoyama Y, Suzuki T, Sakata-Yanagimoto M, et al. Derivation of functional mature neutrophils from human embryonic stem cells. Blood 2009;113:6584-92. [Crossref] [PubMed]
- Klimchenko O, Di Stefano A, Geoerger B, et al. Monocytic cells derived from human embryonic stem cells and fetal liver share common differentiation pathways and homeostatic functions. Blood 2011;117:3065-75. [Crossref] [PubMed]
- Chan KM, Bonde S, Klump H, et al. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood 2008;111:2953-61. [Crossref] [PubMed]
- Yoshimoto M, Heike T, Chang H, et al. Bone marrow engraftment but limited expansion of hematopoietic cells from multipotent germline stem cells derived from neonatal mouse testis. Exp Hematol 2009;37:1400-10. [Crossref] [PubMed]
- Li D, Xue W, Li M, et al. VCAM-1(+) macrophages guide the homing of HSPCs to a vascular niche. Nature 2018;564:119-24. [Crossref] [PubMed]
- Qian H, Georges-Labouesse E, Nystrom A, et al. Distinct roles of integrins alpha6 and alpha4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood 2007;110:2399-407. [Crossref] [PubMed]


