
Cerebrospinal fluid leaks after spine tumor resection: avoidance, recognition and management
Introduction
Cerebrospinal fluid (CSF) leaks are a known complication of spine surgery, and an effective protocol for prevention, recognition and treatment of postoperative CSF leakage is essential to avoiding a cascade of associated adverse outcomes, such as durocutaneous fistula, wound infection (1-5), intracranial hemorrhage (6-12), arachnoiditis (13,14), nerve root incarceration/strangulation (15,16) and meningitis (3-5,17). Unintended durotomies are reported to occur in up to 16% of spine surgeries in several large series (14,15,18-38), with smaller series reporting even higher incidences (39). While unintended durotomies may lead to longer operative times, a delay in mobilization after surgery, and occasional nerve root injury or neurological deficit (30,31), the majority of incidental durotomies are identified and addressed intraoperatively without the need for reoperation or further intervention, and evidence regarding the overall effect of incidental durotomy on long term patient outcomes are conflicting (19,22,40-42). The adverse outcomes of perhaps greater interest are post-operative CSF leaks that require secondary intervention and lead to CSF-leak-related sequelae after spine surgery, as these are a source of considerable patient morbidity and economic burden (43,44).
Patients undergoing surgical intervention for spinal tumors would seem particularly prone to the development of post-operative CSF leaks due to a number of factors, including: (I) the variable presence of an intradural tumor component requiring durotomy as a part of the intervention, (II) deficits in wound healing capability as a result of malnutrition, complex wounds, medical comorbidities, extended use of high-dose steroids or the need for adjuvant chemoradiotherapy, and (III) the use of anterior/ventral approaches to the spine which may create communications between the subarachnoid space and negative pressure potential spaces (e.g., the pleural cavity).
Inappropriately managed CSF leaks subject spine tumor patients to a number of associated complications, lengthened hospital stays, the need for additional interventions, increased health care costs (43,44), and the propensity for tumor seeding (4,45,46). Early identification and treatment of CSF leaks is necessary to avoid compounding the risks of spine tumor surgery and achieving the best possible patient outcomes. The goal of this article will be to review the literature on post-operative CSF leaks after surgical intervention for spinal tumors—with an emphasis on avoidance, recognition and effective management.
Incidence and risk factors
The reported incidence of CSF leak requiring intervention after spinal tumor surgery varies widely (0–28.6%) (3-5,47-67), but a review of the recent literature would suggest that the overall incidence after surgery for both intradural and extradural pathologies is relatively low (68-77) (mean, 6.6%±5.8%; Tables 1,2).
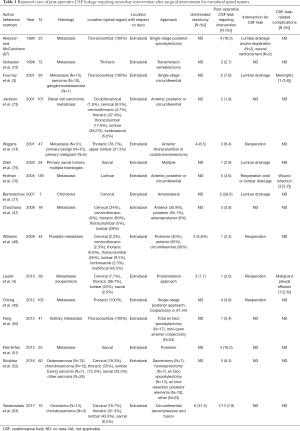
Full table
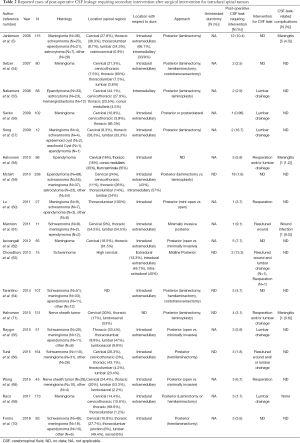
Full table
A number of factors predisposing to unintended durotomy during spine surgery for degenerative pathology have been described, including advanced patient age (26,30,78,79), resident involvement (34,38), history of prior surgery in the same region (36,38,79-81), length of surgery (78), lumbar spine surgery (compared with cervical and thoracic) (38,79), and synovial cyst pathology (30).
Risk factors for the development of post-operative CSF leak requiring intervention after spine surgery, however, are not well defined. In patients undergoing resection of extradural tumors, unintended durotomy is necessary—but not sufficient—for the development of a post-operative CSF leak. Effective closure of dural defects should prevent extradural CSF egress, and wound closure and wound healing are likely fundamental to the prevention of cutaneous CSF fistulae.
Several authors have suggested that minimally invasive surgical (MIS) approaches to spine tumor resection—and other MIS spine surgeries—lead to lower rates of post-operative symptomatic CSF leakage, despite the increased difficulty of primary dural closure through an MIS approach (65,68,70,82-84). This finding is said to be a consequence of the limited soft-tissue exposure and relative absence of “dead space” resulting from MIS approaches, wherein the absence of dead space leads to a relative increase in epidural pressure, “tamponading” the epidural space and preventing epidural CSF egress (84). Applied to open spine tumor resections, these principles would suggest that meticulous closure of not only the dural defect, but of all surgical layers, would decrease the risk of pseudomeningocele development and other manifestations of post-operative CSF leak by decreasing dead space. By extension, perioperative radiotherapy/chemotherapy delivery (85), high-dose steroids, elevated intracranial pressure related to leptomeningeal-disease-related hydrocephalus or intracranial metastases, and comorbidities that delay wound healing would seem likely to elevate the risk of post-operative CSF leak; evidence that speaks to these issues is difficult to find.
Avoidance of unintended durotomy
In intradural spine tumor surgeries, durotomies are inevitable, but avoiding unintended durotomies when resecting epidural spine tumors is the first step in the prevention of post-operative CSF leak. Data from the degenerative spine literature suggest that the Kerrison punch is the tool most likely to cause unintended durotomies, followed by the high-speed drill (26,30). Unintended durotomies have also been reported with greater incidence in surgeries for synovial cysts (30) and revision surgeries (36,38,78-81). Many of these durotomies could likely be prevented through adherence to fundamental surgical principles, such as: (I) adequate visualization of tissues before tissue removal, (II) adequate dissection of tissue planes before tissue removal, and (III) in revision cases or cases in which normal tissue planes are distorted/adherent, beginning with dissection of “normal” tissue planes before proceeding toward adherent/distorted planes.
Epidural spine tumors may be adherent to the dura, distorting normal tissue planes and making dissection difficult. It may be beneficial in these cases to dissect tumor away from the dura in a medial-to-lateral and cranial-to-caudal direction to prevent inadvertent nerve root injury, which could be a source of CSF leak as well as pain or neurological deficit. Additionally, in cases in which a nerve root must be intentionally sacrificed to achieve surgical goals, it is important that this is done in a controlled manner, wherein the root sleeve is ligated securely proximal to the dorsal root ganglion and cut—rather than avulsed—in order to prevent CSF leaks.
Many reported cases of unintended durotomy occur during bony removal. Although a large proportion of unintended durotomies are caused by Kerrison rongeurs (26,30), the high-speed drill is another unfortunate source of CSF leaks and neurological injury. Great caution should of course be taken when using a high-speed drill in all cases, and especially when aggressive, cutting bits are employed. Ultrasonic osteotomes (84) have been purported by some to be less likely to penetrate the spinal dura, but others have found the rate of incidental durotomy with ultrasonic osteotomes to be statistically equivalent to that seen with high-speed drills (86).
Finally, in some cases, post-operative CSF leaks occur in the absence of recognized intraoperative durotomies (6.8–25%) (13,84,87). The underlying cause of such “occult” leaks is unclear. They may be the result of small durotomies occurring intraoperatively with an initially intact arachnoid layer which later ruptures or herniates into the epidural space as an arachnoid-lined, CSF-filled cyst (14). This highlights the importance of diligent inspection of the dura prior to closure. Alternatively, they may occur post-operatively as a result of dural penetration by bony spicules (88,89), in which case they might be prevented by meticulous removal of all epidural protuberances prior to closure.
Recognition of post-operative CSF Leaks
Ideally, recognition of CSF leaks should begin intraoperatively. Unintended durotomies may be recognized not only by the emanation of spinal fluid into the surgical field, by often by a sudden increase in epidural venous bleeding and decrease in thecal sac turgor. In the case of intended durotomies, failure of primary closure may be recognized intraoperatively as persistent CSF egress, a finding that may be elicited more readily with a Valsalva maneuver.
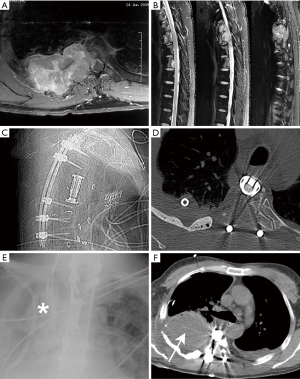
Post-operatively, suspicion for CSF leak may be initially raised by a number of factors, including patient symptoms (e.g., postural headache, axial pain, recurrence of preoperative symptoms), unexpectedly high outputs from wound drainage systems, a palpable fluid collection on physical exam, neurologic deficits (38), or drainage of clear fluid from the wound, any of which may justifiably prompt post-operative imaging (e.g., MRI). The large majority of contained pseudomeningoceles are asymptomatic and likely go unrecognized, but cases of radiculopathy and myelopathy due to compression of neural elements by a pseudomeningocele have been reported (14). Retropleural pseudomeningoceles have also been reported, particularly when transthoracic approaches are used (Figure 1) (90-92).
In cases of post-operative fluid collections or elevated output from subfascial drains of undetermined etiology, testing for beta-2-transferrin, a protein found almost exclusively in CSF, can be used to differentiate post-operative CSF leaks from seromas and other fluid collections (90–94% sensitive, 98–100% specific) (93).
Complications of CSF leak after spine surgery
A multitude of complications have been reported to occur in association with spinal CSF leaks, including infectious complications (e.g., meningitis, arachnoiditis, wound infections), delayed wound healing, complications of intracranial hypotension (e.g., intracranial hemorrhage, cranial nerve palsies), and neurological deficits related to compression or incarceration of neural elements, among others.
Subarachnoid-cutaneous fistulae prevent normal tissue opposition, impair wound healing and also lead to increased risks of meningitis and wound infection (1-5,17), although the precise incidence of these complications in patients with CSF leaks after spine surgery is unclear. Data from the traumatic cranial CSF leak literature report a 10–19% incidence of fulminant meningitis in patients with persistent or occult CSF leakage (94-96), although this may not be generalizable to the spinal surgery patient population. Lin et al. performed a retrospective review of 20,178 patients undergoing spinal surgery at a single institution, reporting that 21 patients (0.10%) developed post-operative meningitis. All 21 patients underwent lumbar spinal surgery for degenerative indications, and incidental durotomy was reported to have occurred in each case, although only 11 of the 21 patients suffered from post-operative CSF leakage, and the incidence of durotomy and/or postoperative CSF leak in the other 20,157 patients was not reported. All 21 patients recovered with antibiotic therapy, although 3 patients required reoperation for repair of the dural defect (17). Arachnoiditis has also been reported to occur in the setting of post-operative CSF leaks, presumably as a consequence of blood products being introduced into the subdural space or infection/inflammation (97). Several authors have also reported nerve root incarceration/strangulation and even spinal cord herniation through a dural defect, leading to pain and neurological deficits (13-16).
Intracranial hypotension is another consequence of persistent CSF leakage, and may lead not only to postural headaches, but also intracranial hemorrhage, cerebellar herniation and cranial nerve deficits (7-12,14,98). Intracranial hemorrhage is thought to occur in the setting of CSF leakage due to CSF hypovolemia and resultant caudally oriented mechanical forces exerted on the brain when in an upright posture, which presumably leads to occlusion or tearing of bridging veins and subsequent venous infarct/hemorrhage (8,10). Cerebellar hemorrhage has been reported to occur with an incidence of 0.8% after all lumbar spine surgeries (i.e., whether complicated by durotomy or not) (12), and poorly controlled CSF diversion through lumbar drains and unmonitoring subfascial drain output in the setting of a durotomy may exacerbate the risk (7).
Tumor seeding is a complication of post-operative CSF leakage unique to patients undergoing resection of malignant spinal tumors (4,45). Tumor seeding is known to occur with CSF diversionary procedures (99), and remote tumor spread via CSF pathways is a known phenomenon in malignant brain tumors and intradural spine tumors (100,101). Such an event has been only rarely reported after extradural spine tumor resection in the setting of an unintended durotomy (46). Currently, the incidence of intradural tumor seeding as a consequence of CSF leak after spine tumor surgery remains unclear.
Finally, there is evidence from several series that CSF leaks may impair bony fusion in spine surgeries for which arthrodesis is a goal, either through the displacement of bone graft or impairment of the cellular signaling cascades necessary for bony growth and fusion (102). Other studies have failed to corroborate this finding, however (19).
Direct repair materials and techniques
Primary repair of durotomies intraoperatively—when feasible—is recommended to prevent post-operative CSF leaks, yet direct durotomy repairs have been reported to fail in 5–9% of cases (103,104). A variety of dural repair materials and techniques may be used depending on surgeon preference, durotomy location and the morphology of the durotomy (e.g., linear tear vs. large defect). Repair of linear, accessible durotomies, including those intentionally created for resection of intradural tumors, is typically undertaken with suture. The comparative effectiveness of various suture materials and techniques in durotomy repair is a somewhat controversial issue, with several studies reporting that an interrupted closure is most effective (105,106), other studies showing similar outcomes with interrupted and running techniques (107,108), and still others reporting that interrupted repairs leak at lower pressures than running-locking repairs (109). While some studies have found that less CSF leakage is seen with prolene suture than silk/nurolon/surgilon (107), others have reported that Gore-Tex suture provides the more watertight closure, owing to the absence of a disparity between the diameters of the suture needle and thread (108). Several authors have reported that in suture with a large needle-to-thread diameter ratio (e.g., prolene, nurolon), CSF leakage is often seen through the suture holes themselves despite an otherwise adequate closure, with nurolon/surgilon leaving a greater dural defect than prolene at the site of the suture hole (107). The needle-to-thread diameter ratio for Gore-Tex suture is close to 1, which is thought to lead to a smaller defect at the suture hole site and less CSF leakage through suture holes (108).
Primary closure of durotomies may be made difficult by durotomy location (e.g., ventral or far-lateral durotomies), presence of a large dural defect, poor tensile strength of the dura, and minimally invasive techniques which limit exposure and access. In cases of far-lateral or ventral durotomies, some authors have advocated the creation of an additional dorsal durotomy, through which the far-lateral defect can be more easily visualized and plugged with autograft (e.g., fat, muscle, fascia) or sutured (14,110). Others have recommended the use of autograft or blood-soaked gelfoam supplemented with a dural sealant in cases of durotomies that cannot be repaired primarily due to limited visibility or access (67,72,103,111,112). Some authors have also reported that titanium clips (68,113), or even aneurysm clips (114), may increase the ease of watertight durotomy closure in cases of minimally invasive or otherwise limited access.
A variety of dural patch graft materials—including autograft, allograft, and both suturable and non-suturable grafts—have been utilized for repair of large dural defects or cases in which the dura cannot be primarily approximated without undue tension on the dural edges or excessive stenosis of the thecal sac. Little consensus or objective evidence exists of one material’s superiority over another, especially with regard to the repair of spinal dural defects (115). A variety of concerns have been raised regarding the use of non-autologous grafts (e.g., allografts, xenografts, synthetic grafts), including graft dissolution, encapsulation, foreign-body or inflammatory reactions, infection, hemorrhage, and excessive scarring and adhesion formation (115-121), while the use of autologous grafts may require an additional incision and additional operative time for harvest and may be of variable suitability and effectiveness in preventing post-operative CSF leaks (122). Although available studies seem to indicate that the risk of wound infection, post-operative CSF leak, and other complications are greater with the use of non-autologous dural substitutes, further study is needed (117,123,124).
Tissue sealants are also frequently used to augment dural repair and decrease dead space within a wound. Animal studies have demonstrated that dural sealants lead to improvements in hydrostatic strength of a primary dural repair (109), but a benefit has not been consistently evidenced in human clinical studies (125-129). A multitude of sealants have been utilized for augmentation of spinal durotomy repair with variable success (25,107), and little evidence exists to recommend one sealant over another. Some authors have reported improved CSF leak indices in patients in whom a primarily repaired durotomy is augmented with polyethylene glycol (PEG) hydrogel sealants as opposed to fibrin glue sealants (130), although certain PEG hydrogel formulations have a documented capacity to expand and cause neural compression (107,131,132).
Wound closure
A meticulous wound closure is an important part of any surgical procedure, but in patients with durotomies, in particular, wound closure may have a considerable effect on the clinical outcome. As mentioned previously, several authors have demonstrated a decreased risk of postoperative CSF leak requiring intervention in patients undergoing minimally invasive resections of intradural spine tumors, a finding perhaps attributable to the differential dead space resulting from open vs. minimally invasive approaches (65,68,70,82-84).
The Hagen-Poiseuille law asserts that the laminar flow of an incompressible, Newtonian fluid with a constant viscosity between two given points is proportional to differential pressures between these two points and the amount of resistance to flow between the points (133). Although the flow of CSF from the subarachnoid space into the extradural space is likely to be turbulent and not entirely laminar, the principles of the law may still provide insight into the pathophysiology of postoperative CSF leaks and the relative success of our interventions to prevent or treat these leaks (103). Reduction of flow through an open dural defect could theoretically be slowed by reduction of intracranial/intrathecal pressure (e.g., treatment of intracranial hypertension or placement of subarachnoid drains), increasing epidural pressure (e.g., by elimination of dead space), or increasing resistance to flow through the defect (e.g., by way of suture, sealants). The goals of wound closure should thus be to eliminate dead space and to create resistance to flow.
The preferred method for elimination of dead space is through meticulous closure of surgical layers. Muscle is the predominant material present in the subfascial space, and thus muscle layers should be approximated, or overlapped through fascial undermining techniques, if feasible. The deep thoracodorsal fascia possesses the greatest tensile strength of all layers closed after spine surgery, and thus provides the greatest resistance to CSF flow. The deep thoracodorsal fascia should be approximately tightly, with closely-spaced, heavy suture (103,134). The skin is not a particularly effective barrier to CSF, and is also highly vascular. Although some have reported success with oversewing a wound after a durocutaneous CSF fistulae develops, the tension that must be placed on the skin in order to prevent CSF flow places the skin edges at risk of ischemia. Others have also reported success with the use of skin sealants (e.g., Dermabond) to arrest a durocutaneous CSF fistulae (135), but in the authors’ experience and that of others (13), this is typically insufficient.
Post-operative positioning
Another controversial matter is that of patient positioning after a durotomy. Conventional wisdom suggests that patient positioning (e.g., flat for lumbar durotomies, upright for cervical durotomies) after a spine surgery complicated by durotomy will decrease the CSF pressure at the site of the durotomy (Bernoulli’s law) (136), thus decreasing the flow of CSF through the dural defect (Poiseuille’s law) (133), but the available evidence is conflicting. A number of authors have reported successful prevention of post-operative CSF leaks requiring intervention after lumbar durotomy when a patient is positioned flat until symptoms (e.g., postural headache) resolve (1–3 days) (25,137-139), but several series have documented similar outcomes with a shortened—or altogether abandoned—period of bedrest (140-143). Given that multiple complications have been reported to result from the aforementioned period of bedrest, including pulmonary, urinary, and cardiac complications, as well as deep venous thrombosis (143), it would seem beneficial for patients to be allowed to mobilize early after a durotomy, but further study regarding the effect on outcomes is needed.
Cerebrospinal fluid diversion
CSF diversion may be employed as a primary (e.g., after a durotomy, in order to prevent post-operative CSF leak) or secondary (after a post-operative CSF leak has developed) intervention for the prevention and treatment of CSF leaks after spine surgery. As discussed previously, Poiseuille’s law dictates that reduction of CSF flow through an open dural defect can be slowed by reduction of intracranial/intrathecal pressure by way of lumbar or intraventricular CSF diversion. In patients with underlying intracranial hypertension, prolonged CSF diversion may be indicated in the form of a ventriculoperitoneal or lumboperitoneal shunt. In many cases, however, temporary drainage (e.g., 5–7 days) via a lumbar subarachnoid catheter is sufficient to allow a dural defect to heal (reported success rate: 85–94%) (103,144,145). The number of days and volume of lumbar drainage required for healing of the dural defect, however, is not well defined. It has been suggested that a drainage volume of 120–360 mL/day for 3–5 days confers a 90–92% success rate in the treatment of a CSF fistula (146), but little high-level evidence exists. Complications associated with lumbar drainage—both minor (e.g., headache, nerve root irritation) and major (e.g., meningitis, intracranial hemorrhage, cranial nerve palsy, retained catheter fragments, spinal hemorrhage) have been reported to occur in up to 44% of cases (103,147-151).
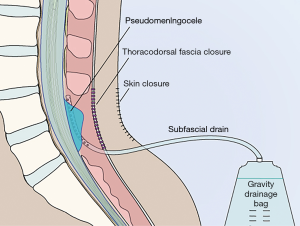
Some authors have reported favorable outcomes with prolonged subfascial/epidural drainage or chest tube drainage (in cases of ventral durotomies after anterior approaches to the thoracic spine) in lieu of a subarachnoid drain (103,152-155). While this would theoretically encourage continued CSF flow through a dural defect, temporary subfascial CSF diversion would allow the fascia time to heal, and after drain removal, the subfascial pressure and intrathecal pressure are said to equalize, leading to indirect slowing of CSF leakage and eventual secondary healing of the dural defect (Figure 2) (152-154). These subfascial drains are typically used without suction (to allow the subarachnoid pressure to dictate the amount of drainage) with the collection bag maintained at the level of the dural defect (to avoid overdrainage by siphoning), although some authors have reported that the use of half-suction or even full suction is relatively safe (153). Several series have suggested the optimal length of subfascial drainage for the prevention of post-operative CSF leak is 7–17 days (152,154).
Epidural blood patch
A number of authors have reported good results with epidural blood patches as a treatment for symptomatic pseudomeningocele after spine surgery (7,93,137,138,156). Similar to the mechanism of action reported for dural sealants, epidural blood patches are said to provide coverage of the dural defect while also filling epidural dead space and increasing epidural pressure, thus providing resistance to continued CSF egress. Some suggest placement of an epidural blood patch adjacent to a recent surgical site to avoid overly deep or superficial injection (93), but ultrasound guidance has also been reported to assist with accurate placement of an epidural blood patch in the setting of post-surgical anatomical changes (156).
Conclusions
Post-operative CSF leak is a known complication of spine surgery. Patients undergoing resection of spine tumors may be particularly susceptible due to number of patient and pathology-related factors. Intraoperative identification of inadvertent durotomies and meticulous primary repair is preferred, but in cases of failed primary repair or unidentified durotomies, early post-operative recognition and secondary intervention may protect patients from CSF-leak-related complications, obviate the need for revision surgery and lead to improved patient outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sun X, Sun C, Liu X, et al. The frequency and treatment of dural tears and cerebrospinal fluid leakage in 266 patients with thoracic myelopathy caused by ossification of the ligamentum flavum. Spine (Phila Pa 1976) 2012;37:E702-7. [Crossref] [PubMed]
- Tosun B, Iibay K, Kim MSM, et al. Management of persistent cerebrospinal fluid leakage following thoraco-lumbar surgery. Asian Spine J 2012;6:157-62. [Crossref] [PubMed]
- Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg 2001;94:232-44. [PubMed]
- Laufer I, Hanover A, Lis E, et al. Repeat decompression surgery for recurrent spinal metastases. J Neurosurg Spine 2010;13:109-15. [Crossref] [PubMed]
- Jenkinson MD, Simpson C, Nicholas RS, et al. Outcome predictors and complications in the management of intradural spinal tumours. Eur Spine J 2006;15:203-10. [Crossref] [PubMed]
- Lu CH, Ho ST, Kong SS, et al. Intracranial subdural hematoma after unintended durotomy during spine surgery. Can J Anaesth 2002;49:100-2. [Crossref] [PubMed]
- Kaloostian PE, Kim JE, Bydon A, et al. Intracranial hemorrhage after spine surgery. J Neurosurg Spine 2013;19:370-80. [Crossref] [PubMed]
- Karaeminogullari O, Atalay B, Sahin O, et al. Remote cerebellar hemorrhage after a spinal surgery complicated by dural tear: Case report and literature review. Neurosurgery 2005;57:E215. [PubMed]
- Yoo JC, Choi JJ, Lee DW, et al. Remote cerebellar hemorrhage after intradural disc surgery. J Korean Neurosurg Soc 2013;53:118-20. [Crossref] [PubMed]
- Friedman JA, Ecker RD, Piepgras DG, et al. Cerebellar hemorrhage after spinal surgery: Report of two cases and literature review. Neurosurgery 2002;50:1361-3. [PubMed]
- Buvanendran A, Byrne RW, Kari M, et al. Occult cervical (C1–2) dural tear causing bilateral recurrent subdural hematomas and repaired with cervical epidural blood patch. J Neurosurg Spine 2008;9:483-7. [Crossref] [PubMed]
- Fernandez-Jara J, Jorge-Blanco A, Carro-Martinez AI, et al. Remote cerebellar hemorrhage after lumbar spinal surgery. Emerg Radiol 2011;18:177-80. [Crossref] [PubMed]
- Clarke MJ, Krauss WE. Cerebrospinal fluid happens. World Neurosurg 2015;83:308-10. [Crossref] [PubMed]
- Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus 2000;9:e5. [Crossref] [PubMed]
- O’Connor D, Maskery N, Griffiths WEG. Pseudomeningocele nerve root entrapment after lumbar discectomy. Spine (Phila Pa 1976) 1998;23:1501-2. [Crossref] [PubMed]
- Oterdoom DLM, Groen RJM, Coppes MH. Cauda equina entrapment in a pseudomeningocele after lumbar Schwannoma extirpation. Eur Spine J 2010;19:S158-61. [Crossref] [PubMed]
- Lin TY, Chen WJ, Hsieh MK, et al. Postoperative meningitis after spinal surgery: A review of 21 cases from 20,178 patients. BMC Infect Dis 2014;14:220. [Crossref] [PubMed]
- Hannallah D, Lee J, Khan M, et al. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am 2008;90:1101-5. [Crossref] [PubMed]
- Elder BD, Theodros D, Sankey EW, et al. Management of Cerebrospinal Fluid Leakage During Anterior Cervical Discectomy and Fusion and Its Effect on Spinal Fusion. World Neurosurg 2016;89:636-40. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Edwards CC, Heller JG, Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy: An independent matched-cohort analysis. Spine (Phila Pa 1976) 2002;27:1168-75. [Crossref] [PubMed]
- Epstein N. Spondylosis and Ossification of the Posterior Longitudinal Ligament: Review of Operative Technique and Assessment Circumferential Procedures. Surg Neurol 2001;55:313-24. [Crossref] [PubMed]
- Wang MC, Chan L, Maiman DJ, et al. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976) 2007;32:342-7. [Crossref] [PubMed]
- Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg 1997;86:990-7. [Crossref] [PubMed]
- Mayr MT, Subach BR, Comey CH, et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg 2002;96:10-6. [PubMed]
- Sin AH, Caldito G, Smith D, et al. Predictive factors for dural tear and cerebrospinal fluid leakage in patients undergoing lumbar surgery. J Neurosurg Spine 2006;5:224-7. [Crossref] [PubMed]
- Ghobrial GM, Theofanis T, Darden BV, et al. Unintended durotomy in lumbar degenerative spinal surgery: a 10-year systematic review of the literature. Neurosurg Focus 2015;39:E8. [Crossref] [PubMed]
- Guerin P, El Fegoun AB, Obeid I, et al. Incidental durotomy during spine surgery: Incidence, management and complications. A retrospective review. Injury 2012;43:397-401. [Crossref] [PubMed]
- Khan MH, Rihn J, Steele G, et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: A review of 3,183 consecutive degenerative lumbar cases. Spine (Phila Pa 1976) 2006;31:2609-13. [Crossref] [PubMed]
- Takahashi Y, Sato T, Hyodo H, et al. Incidental durotomy during lumbar spine surgery: risk factors and anatomic locations. J Neurosurg Spine 2013;18:165-9. [Crossref] [PubMed]
- McMahon P, Dididze M, Levi AD. Incidental durotomy after spinal surgery: a prospective study in an academic institution. J Neurosurg Spine 2012;17:30-6. [Crossref] [PubMed]
- Cammisa FP, Girardi FP, Sangani PK, et al. Incidental durotomy in spine surgery. Spine (Phila Pa 1976) 2000;25:2663-7. [Crossref] [PubMed]
- Kotilainen E, Valtonen S, Carlson CÅ. Microsurgical treatment of lumbar disc herniation: Follow-up of 237 patients. Acta Neurochir (Wien) 1993;120:143-9. [Crossref] [PubMed]
- Rampersaud YR, Moro ERP, Neary MA, et al. Intraoperative adverse events and related postoperative complications in spine surgery: Implications for enhancing patient safety founded on evidence-based protocols. Spine (Phila Pa 1976) 2006;31:1503-10. [Crossref] [PubMed]
- Saxler G, Krämer J, Barden B, et al. The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine (Phila Pa 1976) 2005;30:2298-302. [Crossref] [PubMed]
- Wang JC, Bohlman HH, Riew KD. Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg Am 1998;80:1728-32. [Crossref] [PubMed]
- Syre P, Bohman LE, Baltuch G, et al. Cerebrospinal fluid leaks and their management after anterior cervical discectomy and fusion: A report of 13 cases and a review of the literature. Spine (Phila Pa 1976) 2014;39:E936-43. [Crossref] [PubMed]
- Wolff S, Kheirredine W, Riouallon G. Surgical dural tears: Prevalence and updated management protocol based on 1359 lumbar vertebra interventions. Orthop Traumatol Surg Res 2012;98:879-86. [Crossref] [PubMed]
- Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech 2008;21:116-9. [Crossref] [PubMed]
- Ulrich NH, Burgstaller JM, Brunner F, et al. The impact of incidental durotomy on the outcome of decompression surgery in degenerative lumbar spinal canal stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS) data-a Swiss prospective multi-center cohort study. BMC Musculoskelet Disord 2016;17:170. [Crossref] [PubMed]
- Desai A, Ball PA, Bekelis K, et al. SPORT: Does incidental durotomy affect longterm outcomes in cases of spinal stenosis? Neurosurgery 2015;76:S57-63. [Crossref] [PubMed]
- Adogwa O, Huang MI, Thompson PM, et al. No difference in postoperative complications, pain, and functional outcomes up to 2 years after incidental durotomy in lumbar spinal fusion: A prospective, multi-institutional, propensity-matched analysis of 1,741 patients. Spine J 2014;14:1828-34. [Crossref] [PubMed]
- Jallo J, Pharmd FRE, Baird CJ, et al. The Cost of Cerebral Spinal Fluid Leaks After Spinal Surgery in the USA. In: 2009 Annual Meeting of the Congress of Neurological Surgeons. New Orleans; 2009.
- Weber C, Piek J, Gunawan D. Health care costs of incidental durotomies and postoperative cerebrospinal fluid leaks after elective spinal surgery. Eur Spine J 2015;24:2065-8. [Crossref] [PubMed]
- Clarke MJ, Vrionis FD. Spinal tumor surgery: Management and the avoidance of complications. Cancer Control 2014;21:124-32. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, O’Toole JE. Intradural tumor recurrence after resection of extradural metastasis: a rare but potential complication of intraoperative durotomy. J Neurosurg Spine 2014;20:734-9. [Crossref] [PubMed]
- Chaichana KL, Woodworth GF, Sciubba DM, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 2008;62:683-92. [Crossref] [PubMed]
- Williams BJ, Fox BD, Sciubba DM, et al. Surgical management of prostate cancer metastatic to the spine. J Neurosurg Spine 2009;10:414-22. [Crossref] [PubMed]
- Chong S, Shin SH, Yoo H, et al. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: Prognostic factors for functional outcome and patients’ survival. Spine J 2012;12:1083-92. [Crossref] [PubMed]
- Fang T, Dong J, Zhou X, et al. Comparison of mini-open anterior corpectomy and posterior total en bloc spondylectomy for solitary metastases of the thoracolumbar spine. J Neurosurg Spine 2012;17:271-9. [Crossref] [PubMed]
- Feiz-Erfan I, Fox B, Nader R, et al. Surgical treatment of sacral metastases: indications and results. J Neurosurg Spine 2012;17:285-91. [Crossref] [PubMed]
- Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Clinical, surgical, and molecular prognostic factors for survival after spinal sarcoma resection. Neurosurg Focus 2016;41:E9. [Crossref] [PubMed]
- Yanamadala V, Rozman PA, Kumar JI, et al. Vascularized Fibular Strut Autografts in Spinal Reconstruction after Resection of Vertebral Chordoma or Chondrosarcoma: A Retrospective Series. Neurosurgery 2017;81:156-64. [Crossref] [PubMed]
- Setzer M, Vatter H, Marquardt G, et al. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus 2007;23:E14. [Crossref] [PubMed]
- Nakamura M, Ishii K, Watanabe K, et al. Surgical treatment of intramedullary spinal cord tumors: Prognosis and complications. Spinal Cord 2008;46:282-6. [Crossref] [PubMed]
- Sacko O, Haegelen C, Mendes V, et al. Spinal meningioma surgery in elderly patients with paraplegia or severe paraparesis: A multicenter study. Neurosurgery 2009;64:503-9. [Crossref] [PubMed]
- Song KW, Shin SI, Lee JY, et al. Surgical results of intradural extramedullary tumors. Clin Orthop Surg 2009;1:74-80. [Crossref] [PubMed]
- Halvorsen CM, Kolstad F, Hald J, et al. Long-term outcome after resection of intraspinal ependymomas: Report of 86 consecutive cases. Neurosurgery 2010;67:1622-31. [Crossref] [PubMed]
- McGirt MJ, Garcés-Ambrossi GL, Parker SL, et al. Short-term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: Analysis of 238 patients. Neurosurgery 2010;66:1005-12. [Crossref] [PubMed]
- Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. J Neurosurg Spine 2011;14:758-64. [Crossref] [PubMed]
- Mannion RJ, Nowitzke AM, Efendy J, et al. Safety and efficacy of intradural extramedullary spinal tumor removal using a minimally invasive approach. Neurosurgery 2011;68:208-16. [PubMed]
- Iacoangeli M, Gladi M, Di Rienzo A, et al. Minimally invasive surgery for benign intradural extramedullary spinal meningiomas: Experience of a single institution in a cohort of elderly patients and review of the literature. Clin Interv Aging 2012;7:557-64. [Crossref] [PubMed]
- Chowdhury FH, Haque MR, Sarker MH. High cervical spinal schwannoma; Microneurosurgical management: An experience of 15 cases. Acta Neurol Taiwan 2013;22:59-66. [PubMed]
- Tarantino R, Donnarumma P, Nigro L, et al. Surgery of intradural extramedullary tumors: Retrospective analysis of 107 cases. Neurosurgery 2014;75:509-14. [Crossref] [PubMed]
- Raygor KP, Than KD, Chou D, et al. Comparison of minimally invasive transspinous and open approaches for thoracolumbar intradural-extramedullary spinal tumors. Neurosurg Focus 2015;39:E12. [Crossref] [PubMed]
- Turel MK, D’Souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: an analysis of 164 patients. Neurosurg Focus 2015;39:E9. [Crossref] [PubMed]
- Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg 1996;85:211-20. [Crossref] [PubMed]
- Wong AP, Lall RR, Dahdaleh NS, et al. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus 2015;39:E11. [Crossref] [PubMed]
- Raco A, Pesce A, Toccaceli G, et al. Factors leading to a poor functional outcome in spinal meningioma surgery: Remarks on 173 cases. Neurosurgery 2017;80:602-9. [Crossref] [PubMed]
- Formo M, Halvorsen CM, Dahlberg D, et al. Minimally invasive microsurgical resection of primary, intradural spinal tumors is feasible and safe: A consecutive series of 83 patients. Neurosurgery 2018;82:365-71. [Crossref] [PubMed]
- Halvorsen CM, Rønning P, Hald J, et al. The Long-term Outcome after Resection of Intraspinal Nerve Sheath Tumors: Report of 131 Consecutive Cases. Neurosurgery 2015;77:585-92. [Crossref] [PubMed]
- Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998;89:599-609. [Crossref] [PubMed]
- Jackson RJ, Loh SC, Gokaslan ZL. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg 2001;94:18-24. [PubMed]
- Wiggins GC, Mirza S, Bellabarba C, et al. Perioperative complications with costotransversectomy and anterior approaches to thoracic and thoracolumbar tumors. Neurosurg Focus 2001;11:e4. [Crossref] [PubMed]
- Zileli M, Hoscoskun C, Brastianos P, et al. Surgical treatment of primary sacral tumors: complications associated with sacrectomy. Neurosurg Focus 2003;15:E9. [Crossref] [PubMed]
- Holman PJ, Suki D, McCutcheon I, et al. Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine 2005;2:550-63. [Crossref] [PubMed]
- Barrenechea IJ, Perin NI, Triana A, et al. Surgical management of chordomas of the cervical spine. J Neurosurg Spine 2007;6:398-406. [Crossref] [PubMed]
- Smorgick Y, Baker KC, Herkowitz H, et al. Predisposing factors for dural tear in patients undergoing lumbar spine surgery. J Neurosurg Spine 2015;22:483-6. [Crossref] [PubMed]
- Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: A multivariate analysis. Spine J 2012;12:121-6. [Crossref] [PubMed]
- Kalevski SK, Peev NA, Haritonov DG. Incidental Dural Tears in lumbar decompressive surgery: Incidence, causes, treatment, results. Asian J Neurosurg 2010;5:54-9. [PubMed]
- Morgan-Hough CVJ, Jones PW, Eisenstein SM. Primary and revision lumbar discectomy. A 16-year review from one centre. J Bone Joint Surg Br 2003;85:871-4. [Crossref] [PubMed]
- Pham M, Chang K, Liu J, et al. Minimally Invasive Surgery for Intradural Extramedullary Spinal Tumors: A Comprehensive Review with Illustrative Clinical Cases. World Spinal Column J 2016;7:84-96.
- Shih P, Wong AP, Smith TR, et al. Complications of open compared to minimally invasive lumbar spine decompression. J Clin Neurosci 2011;18:1360-4. [Crossref] [PubMed]
- Wong AP, Shih P, Smith TR, et al. Comparison of symptomatic cerebral spinal fluid leak between patients undergoing minimally invasive versus open lumbar foraminotomy, discectomy, or laminectomy. World Neurosurg 2014;81:634-40. [Crossref] [PubMed]
- Yokogawa N, Murakami H, Demura S, et al. Postoperative Cerebrospinal Fluid Leakage Associated With Total En Bloc Spondylectomy. Orthopedics 2015;38:e561-6. [Crossref] [PubMed]
- Bydon M, Xu R, Papademetriou K, et al. Safety of spinal decompression using an ultrasonic bone curette compared with a high-speed drill: outcomes in 337 patients. J Neurosurg Spine 2013;18:627-33. [Crossref] [PubMed]
- Gerardi F, Cammisa F, Sangani P. Frequency and sequelae of incidental durotomy. In: The 14th Annual Meeting of the North American Spine Society. Chicago, IL: North American Spine Society, 1999.
- Brookfield K, Randolph J, Eismont F, et al. Delayed symptoms of cerebrospinal fluid leak following lumbar decompression. Orthopedics 2008;31:816. [Crossref] [PubMed]
- Bosacco SJ, Gardner MJ, Guille JT. Evaluation and treatment of dural tears in lumbar spine surgery: A review. Clin Orthop Relat Res 2001.238-47. [Crossref] [PubMed]
- Raffa SJ, Benglis DM, Levi AD. Treatment of a persistent iatrogenic cerebrospinal fluid-pleural fistula with a cadaveric dural-pleural graft. Spine J 2009;9:e25-9. [Crossref] [PubMed]
- Howard BA, Gray L, Isaacs RE, et al. Definitive diagnosis of cerebrospinal fluid leak into the pleural space using 111in-DTPA cisternography. Clin Nucl Med 2015;40:220-3. [Crossref] [PubMed]
- Nairus JG, Richman JD, Douglas RA. Retroperitoneal pseudomeningocele complicated by meningitis following a lumbar burst fracture: A case report. Spine (Phila Pa 1976) 1996;21:1090-3. [Crossref] [PubMed]
- Hershman S, Cuellar VG, Bendo JA. Delayed presentation of incidental durotomy. Bull Hosp Jt Dis (2013) 2013;71:231-4. [PubMed]
- Choi D, Spann R. Traumatic cerebrospinal fluid leakage: Risk factors and the use of prophylactic antibiotics. Br J Neurosurg 1996;10:571-5. [Crossref] [PubMed]
- Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg 2001;25:1062-6. [Crossref] [PubMed]
- Swift AC, Foy P. Advances in the management of CSF rhinorrhoea. Hosp Med 2002;63:28-32. [Crossref] [PubMed]
- Whetstone KE, Crane DA. Cauda equina syndrome resulting from lumbar arachnoiditis after intracranial subarachnoid hemorrhage: A case report. PM R 2013;5:539-41. [Crossref] [PubMed]
- Andrews RT, Koci TM. Cerebellar herniation and infarction as a complication of an occult postoperative lumbar dural defect. Am J Neuroradiol 1995;16:1312-5. [PubMed]
- Han YP, Zhao Y, He XG, et al. Peritoneal metastasis of third ventricular atypical teratoid/rhabdoid tumor after VP shunt implantation for unexplained hydrocephalus. World J Pediatr 2012;8:367-70. [Crossref] [PubMed]
- Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 2008;108:248-57. [Crossref] [PubMed]
- Robles SG, Saldaña C, Boto GR, et al. Intradural Extramedullary Spinal Ependymoma: A Benign Pathology? Spine (Phila Pa 1976) 2005;30:E251-4. [Crossref] [PubMed]
- Bydon M, De La Garza-Ramos R, Abt NB, et al. Durotomy is associated with pseudoarthrosis following lumbar fusion. J Clin Neurosci 2015;22:544-8. [Crossref] [PubMed]
- Fang Z, Tian R, Jia YT, et al. Treatment of cerebrospinal fluid leak after spine surgery. Chin J Traumatol 2017;20:81-3. [Crossref] [PubMed]
- Narotam PK, José S, Nathoo N, et al. Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique. Spine (Phila Pa 1976) 2004;29:2861-7. [Crossref] [PubMed]
- Chen YX, Chen LE, Seaber A V., et al. Comparison of continuous and interrupted suture techniques in microvascular anastomosis. J Hand Surg Am 2001;26:530-9. [Crossref] [PubMed]
- Megyesi JF, Ranger A, MacDonald W, et al. Suturing technique and the integrity of dural closures: An in vitro study. Neurosurgery 2004;55:950-4. [Crossref] [PubMed]
- Dafford EE, Anderson PA. Comparison of dural repair techniques. Spine J 2015;15:1099-105. [Crossref] [PubMed]
- Ghobrial GM, Maulucci CM, Viereck MJ, et al. Suture Choice in Lumbar Dural Closure Contributes to Variation in Leak Pressures: Experimental Model. Clin Spine Surg 2017;30:272-5. [Crossref] [PubMed]
- Cain JE, Rosenthal HG, Broom MJ, et al. Quantification of leakage pressures after durotomy repairs in the canine. Spine (Phila Pa 1976) 1990;15:969-70. [Crossref] [PubMed]
- Lee DH, Kim KT, Park JI, et al. Repair of Inaccessible Ventral Dural Defect in Thoracic Spine: Double Layered Duraplasty. Korean J Spine 2016;13:87. [Crossref] [PubMed]
- Black P. Cerebrospinal fluid leaks following spinal or posterior fossa surgery: use of fat grafts for prevention and repair. Neurosurg Focus 2000;9:e4. [Crossref] [PubMed]
- Ruban D, O’Toole JE. Management of incidental durotomy in minimally invasive spine surgery. Neurosurg Focus 2011;31:E15. [Crossref] [PubMed]
- Faulkner ND, Finn MA, Anderson PA. Hydrostatic comparison of nonpenetrating titanium clips versus conventional suture for repair of spinal durotomies. Spine (Phila Pa 1976) 2012;37:E535-9. [Crossref] [PubMed]
- Beier AD, Barrett RJ, Soo TM. Aneurysm Clips for Durotomy Repair. Oper Neurosurg (Hagerstown) 2010;66:ons-E124-ons-E125.
- Abla AA, Link T, Fusco D, et al. Comparison of dural grafts in Chiari decompression surgery: Review of the literature. J Craniovertebr Junction Spine 2010;1:29-37. [Crossref] [PubMed]
- Bejjani GK, Zabramski J. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J Neurosurg 2007;106:1028-33. [Crossref] [PubMed]
- Malliti M, Page P, Gury C, et al. Comparison of Deep Wound Infection Rates Using a Synthetic Dural Substitute (Neuropatch) or Pericranium Graft for Dural Closure: A Clinical Review of 1 Year. Neurosurgery 2004;54:599-603. [Crossref] [PubMed]
- Warren WL, Medary MB, Dureza CD, et al. Dural repair using acellular human dermis: Experience with 200 cases: Technique assessment. Neurosurgery 2000;46:1391-6. [Crossref] [PubMed]
- Siccardi D, Ventimiglia A. Fibrotic-haemorrhagic reaction to synthetic dural substitute. Acta Neurochir (Wien) 1995;132:148-9. [Crossref] [PubMed]
- Azzam D, Romiyo P, Nguyen T, et al. Dural Repair in Cranial Surgery Is Associated with Moderate Rates of Complications with Both Autologous and Nonautologous Dural Substitutes. World Neurosurg 2018;113:244-8. [Crossref] [PubMed]
- Foy AB, Giannini C, Raffel C. Allergic reaction to a bovine dural substitute following spinal cord untethering. J Neurosurg Pediatr 2008;1:167-9. [Crossref] [PubMed]
- Attenello FJ, McGirt MJ, Garcés-Ambrossi GL, et al. Suboccipital decompression for Chiari I malformation: Outcome comparison of duraplasty with expanded polytetrafluoroethylene dural substitute versus pericranial autograft. Childs Nerv Syst 2009;25:183-90. [Crossref] [PubMed]
- Raul J-S, Godard J, Arbez-Gindre F, et al. Use of polyester urethane (Neuro-Patch®) as a dural substitute. Prospective study of 70 cases. Neurochirurgie 2003;49:83-9. [PubMed]
- Stendel R, Danne M, Fiss I, et al. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg 2008;109:215-21. [Crossref] [PubMed]
- Nakamura H, Matsuyama Y, Yoshihara H, et al. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: a randomized controlled trial. Spine (Phila Pa 1976) 2005;30:E347-51. [Crossref] [PubMed]
- Yoshimoto T, Sawamura Y, Houkin K, et al. Effectiveness of Fibrin Glue for Preventing Postoperative Extradural Fluid Leakage. Neurol Med Chir (Tokyo) 1997;37:886-9. [Crossref] [PubMed]
- Osbun JW, Ellenbogen RG, Chesnut RM, et al. A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (duraseal dural sealant system) as a dural sealant in cranial surgery. World Neurosurg 2012;78:498-504. [Crossref] [PubMed]
- Than KD, Baird CJ, Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery 2008;63:ONS182-6. [PubMed]
- Jankowitz BT, Atteberry DS, Gerszten PC, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J 2009;18:1169-74. [Crossref] [PubMed]
- Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal spinal sealant) as an adjunct to sutured dural repair in the spine: Results of a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976) 2011;36:1906-12. [Crossref] [PubMed]
- Thavarajah D, De Lacy P, Hussain R, et al. Postoperative cervical cord compression induced by hydrogel (DuraSeal): A possible complication. Spine (Phila Pa 1976) 2010;35:E25-6. [Crossref] [PubMed]
- Lee SH, Park CW, Lee SG, et al. Postoperative Cervical Cord Compression Induced by Hydrogel Dural Sealant (DuraSeal®). Korean J Spine 2013;10:44-6. [Crossref] [PubMed]
- Sutera SP, Skalak R. The History of Poiseuille’s Law. Annu Rev Fluid Mech 1993;25:1-20. [Crossref]
- Steinmetz MP, Benzel EC. Benzel’s spine surgery : techniques, complication avoidance, and management. Elsevier; 4 edition 2017. 5446 p.
- Rotenberg BW, Marchie A, Cusimano MD. Skin sealants: An effective option for closing cerebospinal fluid leakage. Can J Surg 2004;47:466-8. [PubMed]
- Young D, Munson B, Okiishi T, et al. A Brief Introduction to Fluid Mechanics. John Wiley & Sons, Inc., 2003.
- Menon SK, Onyia CU. A short review on a complication of lumbar spine surgery: CSF leak. Clin Neurol Neurosurg 2015;139:248-51. [Crossref] [PubMed]
- Lotfinia I, Sayyahmelli S. Incidental durotomy during lumbar spine surgery. Neurosurg Q 2012;22:105-12. [Crossref]
- Grannum S, Patel MS, Attar F, et al. Dural tears in primary decompressive lumbar surgery. Is primary repair necessary for a good outcome? Eur Spine J 2014;23:904-8. [Crossref] [PubMed]
- Hodges SD, Humphreys SC, Eck JC, et al. Management of incidental durotomy without mandatory bed rest: A retrospective review of 20 cases. Spine (Phila Pa 1976) 1999;24:2062-4. [Crossref] [PubMed]
- Low JCM, Von Niederhäusern B, Rutherford SA, et al. Pilot study of perioperative accidental durotomy: Does the period of postoperative bed rest reduce the incidence of complication? Br J Neurosurg 2013;27:800-2. [Crossref] [PubMed]
- Bonanos G, Coulter I, Prasad M. Bed rest and beyond: An audit of bed rest for managing incidental durotomy in lumbar spine surgery. In: Proceedings of the 2015 Autumn Meeting of the Society of British Neurological Surgeons 2015:472-3.
- Radcliff KE, Sidhu GDS, Kepler CK, et al. Complications of Flat Bedrest Following Incidental Dural Repair. Clin Spine Surg 2016;29:281-4. [Crossref] [PubMed]
- Kitchel SH, Eismont FJ, Green BA. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg Am 1989;71:984-7. [Crossref] [PubMed]
- Farhat HI, Elhammady MS, Levi AD, et al. Cervical subarachnoid catheter placement for continuous cerebrospinal fluid drainage: A safe and efficacious alternative to the classic lumbar cistern drain. Neurosurgery 2011;68:52-6. [PubMed]
- Chaudhry S, Ishaque M. A simple lumbar drainage system. Tech Orthop 2012;27:265. [Crossref]
- AçIkbaş SC, Akyüz M, Kazan S, et al. Complications of closed continuous lumbar drainage of cerebrospinal fluid. Acta Neurochir (Wien) 2002;144:475-80. [Crossref] [PubMed]
- Dardik A, Perler BA, Roseborough GS, et al. Subdural hematoma after thoracoabdominal aortic aneurysm repair: An underreported complication of spinal fluid drainage? J Vasc Surg 2002;36:47-50. [Crossref] [PubMed]
- Coplin WM, Avellino AM, Kim DK, et al. Bacterial meningitis associated with lumbar drains: A retrospective cohort study. J Neurol Neurosurg Psychiatry 1999;67:468-73. [Crossref] [PubMed]
- Cheung AT, Pochettino A, Guvakov DV, et al. Safety of lumbar drains in thoracic aortic operations performed with extracorporeal circulation. Ann Thorac Surg 2003;76:1190-6. [Crossref] [PubMed]
- Weaver KD, Wiseman DB, Farber M, et al. Complications of lumbar drainage after thoracoabdominal aortic aneurysm repair. J Vasc Surg 2001;34:623-7. [Crossref] [PubMed]
- Fang Z, Jia YT, Tian R, et al. Subfascial drainage for management of cerebrospinal fluid leakage after posterior spine surgery - A prospective study based on Poiseuille’s law. Chin J Traumatol 2016;19:35-8. [Crossref] [PubMed]
- Niu T, Lu DS, Yew A, et al. Postoperative Cerebrospinal Fluid Leak Rates with Subfascial Epidural Drain Placement after Intentional Durotomy in Spine Surgery. Global Spine J 2016;6:780-5. [Crossref] [PubMed]
- Hughes SA, Ozgur BM, German M, et al. Prolonged Jackson-Pratt drainage in the management of lumbar cerebrospinal fluid leaks. Surg Neurol 2006;65:410-4. [Crossref] [PubMed]
- Cho JY, Chan CK, Lee SH, et al. Management of cerebrospinal fluid leakage after anterior decompression for ossification of posterior longitudinal ligament in the thoracic spine: The utilization of a volume-controlled pseudomeningocele. J Spinal Disord Tech 2012;25:E93-102. [Crossref] [PubMed]
- Fridley JS, Jea A, Glover CD, et al. Symptomatic postsurgical cerebrospinal fluid leak treated by aspiration and epidural blood patch under ultrasound guidance in 2 adolescents: Report of 2 cases. J Neurosurg Pediatr 2013;11:87-90. [Crossref] [PubMed]


