
Trochanteric pressure ulcers: preoperative management and reconstructive considerations
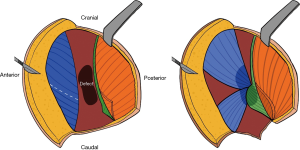
Tan et al. present an interesting case of a trochanteric pressure ulcer repair (1). The reconstruction utilized three local flaps to cover the defect exposed over the greater trochanter. Most of the greater trochanter was covered with a tensor fascia latae myocutaneous flap, while the inferior portion of the bone was covered with two fasciocutaneous flaps (Figure 1). The authors stressed that thorough wound debridement and dead space elimination are the most crucial components of pressure ulcer repair and we agree with this fundamental principle for successful pressure ulcer reconstruction. In this editorial we will highlight the important aspects of trochanteric pressure ulcer management and identify areas deserving of greater collective attention.
Preoperative management
Pressure ulcers affect up to 30% of people in healthcare facilities around the world (2,3). Patients with spinal cord injury comprise a significant portion of this population, with lifetime incidence of pressure ulcers reaching 86% in paraplegic patients (4). As patients with spinal cord injury are prone to developing infection due to their catabolic state, prompt management of pressure ulcers is critical to limit infectious complications. Preoperative care should include nutritional rehabilitation, local wound care, and infection control. Muscle spasm control with antispasmodic agents can limit postoperative flap dehiscence, and surgical correction of contractures prior to reconstruction facilitates optimal patient positioning (5,6).
While the authors do not specifically elucidate the measures taken to rule out osteomyelitis, we assume this was done, and emphasize that infection control is a key component of the preoperative management of pressure ulcers. Plain radiographs are inexpensive and have high specificity for diagnosing osteomyelitis, but they lack sensitivity. Magnetic resonance imaging (MRI) is considered the preferred imaging modality as it has high sensitivity and specificity. However, recent data suggests that diagnosing osteomyelitis preoperatively with an MRI, as opposed to intraoperatively, does not result in improved surgical outcomes in patients with pressure ulcers (7). Intraoperative bone biopsy for culture and histopathology therefore remains the gold standard for diagnosing osteomyelitis, and should be performed in all pressure ulcer cases with suspected joint capsule or bone involvement (5). In cases of trochanteric pressure ulcers like the one described, it is our practice to aggressively debride the greater trochanter to allow tissue sampling from a clean wound. If bone infection is present, then the Girdlestone procedure, which consists of femoral head ostectomy followed by muscle flap closure of the resulting defect, is considered as it has demonstrated benefits in spinal cord injury patients (8,9).
Reconstructive considerations
For advanced stage III and stage IV pressure ulcers like the trochanteric ulcer described, the mainstay of surgical management consists of wound debridement and subsequent soft tissue coverage. Tan et al. emphasize the importance of multiple rounds of debridement and drainage, which rid the wound of inflammatory debris and necrotic tissue that would otherwise impede wound healing and flap survival (1).
Different types of flaps are available in the plastic surgeon’s arsenal for the repair of trochanteric pressure ulcers. These include the tensor fascia latae (TFL) flap designed as a V-Y advancement or rotation flap, the anterolateral thigh (ALT) flap, the distal gluteus maximus myocutaneous flap, the vastus lateralis muscle flap, and the rectus femoris muscle flap (6,8,10-12). The most frequently used flap in the management of trochanteric pressure injury is the TFL flap, which has been modified over time to improve wound coverage and maximize flap survival. The TFL flap can be designed with a hatchet-shaped incision to provide more well-vascularized muscle to fill the wound defect, or combined with tangential splitting of the vastus lateralis to increase flap distance (13-15). Another commonly used flap for trochanteric reconstruction is the pedicled ALT flap, which has lower rates of ulcer recurrence compared to the hatchet-shaped TFL flap (16).
Tan et al. present an interesting reconstructive approach in this case (1). The choice to use a muscle flap was appropriate, as the bulk provided by muscle tissue allows for complete coverage of the defect and filling of potential spaces, thereby limiting seroma, hematoma or abscess formation that would compromise flap survival. The flap design reduces donor site morbidity, and the TFL is a robust muscle supplied by anterior and lateral arterial branches that allow for good vascularized wound coverage. Careful examination of the intraoperative pictures presented in the case reveals that the 8 cm × 10 cm composite tissue flap described might potentially not come from the TFL, but rather the gluteus. Based on patient positioning, it looks as though the muscle flap isolated in Figure 3 originates more posteriorly than where one would expect to find the belly of the TFL, which runs laterally from its attachment on the anterior superior iliac spine to the iliotibial band. Moreover, paraplegic patients often have atrophic TFL muscles from disuse, unlike the muscle pictured in the case. Another possibility is that the flap in question is of hybrid origin, containing muscle fibers of both the TFL and the gluteus.
Another important consideration for pressure ulcer surgery is attempting to preserve local tissue for future reconstructive attempts, given the high rate of ulcer recurrence in the paraplegic patient population. The authors employ this principle by using local myocutaneous and fasciocutaneous flaps without significantly compromising tissue integrity in case a future reconstruction is warranted (1). This design also preserves tissue for subsequent reconstruction of the patient’s sacral ulcer. In patients with multiple pressure ulcers such as this one, consideration should be given to performing a single-stage procedure, as it results in shorter hospital stay and fewer anesthetic procedures for the patient compared to multiple-stage reconstruction (6,17). A reasonable option for coverage of a trochanteric ulcer coexisting with another pressure ulcer is the distal gluteus maximus myocutaneous flap, which could have been leveraged for simultaneous reconstruction of the patient’s two ulcers (18). Finally, although a skin defect is appreciated 3 weeks postoperatively, this complication is not uncommon as the frequency of suture line dehiscence in pressure ulcer reconstruction can be as high as 31% (19-21).
In conclusion, preoperative management of pressure ulcers such as the one described relies on thorough bone debridement with workup for osteomyelitis. Although trochanteric pressure ulcers can be suited for primary repair, many reconstructive considerations should be taken into account to achieve optimal tissue coverage including size, location and amount of pressure ulcer, quantity and quality of local tissue available, and probability that a future reconstruction may be required.
Acknowledgements
The authors would like to thank Salah Rubayi, MD, for his expertise and discussion of this case.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tan J, Chen C, Zhang M. Primary repair of a massive pressure ulcer on the hip: report of one case. Ann Transl Med 2018;6:361. [Crossref] [PubMed]
- Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage 2008;54:40-54. [PubMed]
- Chen HL, Shen WQ, Liu P. A Meta-analysis to Evaluate the Predictive Validity of the Braden Scale for Pressure Ulcer Risk Assessment in Long-term Care. Ostomy Wound Manage 2016;62:20-8. [PubMed]
- Sumiya T, Kawamura K, Tokuhiro A, et al. A survey of wheelchair use by paraplegic individuals in Japan. Part 2: Prevalence of pressure sores. Spinal Cord 1997;35:595-8. [Crossref] [PubMed]
- Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J. Spinal Cord Med 2013;36:572-85. [Crossref] [PubMed]
- Rubayi S. Reconstructive Plastic Surgery of Pressure Ulcers. Berlin, Heidelberg: Springer Berlin Heidelberg, 2015.
- Daniali LN, Keys K, Katz D, et al. Effect of preoperative magnetic resonance imaging diagnosis of osteomyelitis on the surgical management and outcomes of pressure ulcers. Ann Plast Surg 2011;67:520-5. [Crossref] [PubMed]
- Rubayi S, Chandrasekhar BS. Trunk, abdomen, and pressure sore reconstruction. Plast Reconstr Surg 2011;128:201e-15e. [Crossref] [PubMed]
- Rubayi S, Pompan D, Garland D. Proximal femoral resection and myocutaneous flap for treatment of pressure ulcers in spinal injury patients. Ann Plast Surg 1991;27:132-8. [Crossref] [PubMed]
- Foster RD, Anthony JP, Mathes SJ, et al. Flap selection as a determinant of success in pressure sore coverage. Arch Surg 1997;132:868-73. [Crossref] [PubMed]
- Wang CH, Chen SY, Fu JP, et al. Reconstruction of trochanteric pressure sores with pedicled anterolateral thigh myocutaneous flaps. J Plast Reconstr Aesthet Surg 2011;64:671-6. [Crossref] [PubMed]
- Nahai F, Silverton JS, Hill HL, et al. The tensor fascia lata musculocutaneous flap. Ann Plast Surg 1978;1:372-9. [Crossref] [PubMed]
- Demirseren ME, Gökrem S, Ozdemir OM, et al. Hatchet-shaped tensor fascia lata musculocutaneous flap for the coverage of trochanteric pressure sores: a new modification. Ann Plast Surg 2003;51:419-22. [Crossref] [PubMed]
- Jósvay J, Sashegyi M, Kelemen P, et al. Modified tensor fascia lata musculofasciocutaneous flap for the coverage of trochanteric pressure sores. J Plast Reconstr Aesthet Surg 2006;59:137-41. [Crossref] [PubMed]
- Lin MT, Chang KP, Lin SD, et al. Tensor fasciae latae combined with tangentially split vastus lateralis musculocutaneous flap for the reconstruction of pressure sores. Ann Plast Surg 2004;53:343-7. [Crossref] [PubMed]
- Li CC, Chang SC, Fu JP, et al. Comparison of hatchet-shaped tensor fascia lata flap and pedicle anterior lateral thigh flap for treatment of trochanteric sores: a retrospective analysis of 48 patients. Ann Plast Surg 2013;71:659-63. [Crossref] [PubMed]
- Rubayi S, Burnett CC. The efficacy of single-stage surgical management of multiple pressure sores in spinal cord-injured patients. Ann Plast Surg 1999;42:533-9. [Crossref] [PubMed]
- Nisanci M, Sahin I, Eski M, et al. A new flap alternative for trochanteric pressure sore coverage: distal gluteus maximus musculocutaneous advancement flap. Ann Plast Surg 2015;74:214-9. [Crossref] [PubMed]
- Schryvers OI, Stranc MF, Nance PW. Surgical treatment of pressure ulcers: 20-year experience. Arch Phys Med Rehabil 2000;81:1556-62. [Crossref] [PubMed]
- Srivastava A, Gupta A, Taly AB, et al. Surgical management of pressure ulcers during inpatient neurologic rehabilitation: outcomes for patients with spinal cord disease. J Spinal Cord Med 2009;32:125-31. [Crossref] [PubMed]
- Thomson CH, Choudry M, White C, et al. Multi-disciplinary management of complex pressure sore reconstruction: 5-year review of experience in a spinal injuries centre. Ann R Coll Surg Engl 2017;99:169-74. [Crossref] [PubMed]


