
Hyaluronic acid-induced diffuse alveolar hemorrhage: unknown complication induced by a well-known injectable agent
Introduction
Hyaluronic acid (HA), a ubiquitous glycosaminoglycan composed of repeating disaccharide D-glucuronic acid and N-acetyl-D-glucosamine, is widely distributed throughout human body including joints, eyeballs, and connective tissues (1,2). HA is involved in various physiological functions ranging from wound healing to coagulation (2-4). Owing to its high biocompatibility and non-immunogenicity, HA is widely used throughout the medical field, especially as an injectable dermal filler for cosmetic purpose (5). Gaining popularity over the past two decades, HA has become the top injectable soft tissue filler agent around the world; more than 1.8 million procedures were performed in 2014 (5). Although a variety of HA fillers are commercially available and they differ in HA concentration, particle size, cross linking density, and hydration, HA dermal injection has been regarded as safe in the hands of experienced medical personnel (6). Most HA injection-related adverse effects are known to be local reactions including bruise, erythema, swelling and sometimes more severe events such as tissue necrosis and infection (7,8). Pulmonary complications related to HA injection have been scarcely reported. Most of them are non-thrombotic pulmonary embolisms (NTPEs) (9-12) and only one case of HA-related DAH has been previously published so far (13). Here, we report an interesting case of diffuse alveolar hemorrhage (DAH) that occurred in a woman who received illegal vaginal injection of HA.
Case presentation
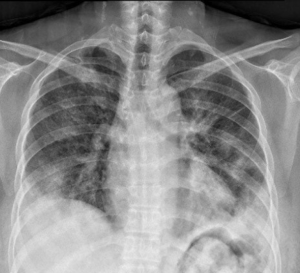
A 56-year-old female visited our pulmonary clinic complaining of hemoptysis and shortness of breath starting a week ago. She had been treated for pulmonary tuberculosis 40 years ago and was taking statin and antidepressant from a local clinic for several years. She has smoked one-pack of cigarette per day for 35 years and drunk alcohol 2 or 3 times per week. She looked acutely ill and tachypneic. Her blood pressure, heart rate and respiration rate were 100/60 mmHg, 98 beats per minute and 22 breaths per minute, respectively. In room air, oxygen saturation was 89% and partial pressure of oxygen in the arterial blood was 72 mmHg. Physical examination was non-significant except bilateral crackle on chest auscultation. Chest X-ray showed bilateral diffuse ground-glass opacities (GGOs) and consolidations (Figure 1). Chest computed tomographic (CT) scan showed diffuse GGOs with interlobular septal thickening and irregular subpleural consolidation in both lower lobes without mediastinal lymphadenopathy or pleural effusion (Figure 2A). There was no definite evidence of pulmonary thromboembolism or right ventricular enlargement. Laboratory test revealed white blood cell count of 9,680/µL (neutrophil 70.9% and lymphocyte 19.5%), hemoglobin of 10.7 g/dL, platelet count of 284,000/µL, C-reactive protein level of 3.92 mg/dL (normal range <0.5 mg/dL), INR of prothrombin time of 1.0, and d-dimer of 0.18 µg/mL. Aspartate aminotransferase, alanine aminotransferase, and total bilirubin levels were elevated to 198 U/L, 228 U/L, and 1.36 mg/dL, respectively.
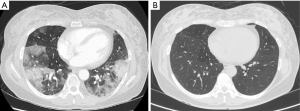
After supplemental oxygen via nasal cannula (3 L/min), she was clinically stabilized. Based on the location of GGOs in the chest CT scan, bronchoalveolar lavage (BAL) was performed on the following day for the superior segments of both lower lobes, which revealed fluid with hemorrhagic features in sequential samples compatible with DAH. Pulmonary function test showed a mild restrictive pattern; forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were 73% and 70% of predicted value, respectively, with FEV1/FVC of 84%. Diffusing capacity of carbon monoxide was 114% of predicted value. We started intravenous methylprednisolone (1 mg/kg per 24 hours) as a treatment of DAH after performing serologic tests for hidden connective tissue diseases or viral diseases to identify the underlying cause of DAH. However, autoantibodies including anti-nuclear, anti-neutrophil cytoplasmic, anti-glomerular basement membrane, anti-citrullinated protein antibodies, and rheumatoid factor, antibodies for viral hepatitis, and antibody for human immunodeficiency virus were all negative. Microbiological test including viral and fungal pathogens, and cytological examination using BAL fluid were all negative. Transthoracic echocardiography revealed normal cardiac function without valvular abnormality. Abdominal ultrasonography showed heterogeneous hepatic echogenicity compatible with chronic alcoholic liver disease. During detailed history taking, she recalled that she was injected with dermal filler around her vaginal wall by an unlicensed medical practitioner on the day before the symptom onset. The agent was commercially available HA filler, and 1 mL of HA was injected 15 times (a total of 15 mL). Thus, we postulated that HA dermal filler injection was the possible cause of DAH. Her respiratory symptoms and radiological abnormalities were gradually improved after the treatment. On the fifth day of the treatment, she was discharged with 20 mg of oral prednisolone. Follow-up chest CT scan taken in the outpatient clinic showed total resolution of previous lung lesions (Figure 2B).
Discussion
DAH is characterized by the disruption of the alveolar-capillary basement membrane caused by damage or inflammation of arterioles, venules, or alveolar septal capillaries (14). Hemoptysis, cough, and dyspnea are common symptoms, however, hemoptysis could be absent even in the case of extensive hemorrhage (13,15). Although chest imaging, decreased hemoglobin or hematocrit level, and increased diffusing capacity can support the diagnosis of DAH, BAL is the crucial step for the diagnosis and differentiation from other lung diseases including eosinophilic pneumonia, hypersensitivity pneumonitis and acute interstitial pneumonia (14). Once DAH is diagnosed, underlying etiology should be searched for successful treatment. In the present case, we could rule out other possible causes such as medications or connective tissue diseases through physical examination and laboratory tests. Through detailed history taking, we had to suspect the causal relationship between HA injection and DAH. Although we could not exclude the possibility that the procedure itself, or an additive or a contaminant of the HA preparation might be the cause, it was most reasonable explanation that excessive HA injected by nonmedical personnel was the culprit of the development of DAH.
Initially, HA injection was introduced in the medical field as joint fluid replacement to treat osteoarthritis or vitreous replacement in ophthalmologic surgery (16,17). HA-based dermal fillers were first available in Europe in 1996 and their usage for cosmetic purposes was approved by the US FDA in 2004 (5). Among many cosmetic injectables including collagen, synthetic polymers, and calcium hydroxyapatite, HA is most preferred and most widely used around the world due to its non-immunogenicity and longevity within tissues (18). HA injection has been regarded to be relatively safe as most adverse effects are local reactions including bruise, swelling, granuloma formation, and sometimes tissue necrosis or vascular occlusion (19). Pulmonary complications of HA injection were scarcely reported; only five cases had been reported so far (4,9-11,13). As summarized in Table 1, most of the pulmonary complications are NTPEs; two cases were intra-articular injection-related and three were dermal or mucosal injection-related. In those cases, respiratory symptoms developed within several hours to days after HA injection. To the best of our knowledge, this is the first case showing typical DAH manifestation after vaginal injection of HA. Interestingly, Park et al. (9) have reported a case of NTPE occurring in a middle-aged female after illegal vaginal injection of HA by unlicensed personnel, very similar to our case. Filler injection into vaginal wall is medically non-indicated because of insufficient efficacy and safety (19). Basora et al. have described a DAH case occurring in a young male after injection of HA into his calf (13). Similar to our present case, his symptoms started the day after HA injection and the volume of injected HA was 50 mL which was far more than the usual dose. Despite DAH was so extensive that the patient had to be applied with non-invasive ventilation for severe hypoxemia, he only complained dry cough and dyspnea without hemoptysis (13).

Full table
The underlying mechanism of HA-induced DAH is currently unclear. However, aberrancy in normal hemostasis caused by HA might be one of possible explanations. Although HA filler has been regarded as a static material and simply occupying space within the tissue, investigations has revealed that HA is osmotically active. Thus it can retract water from surrounding normal tissues (20). In addition, HA stimulates fibroblast, resulting in deposition of type I and type III collagen (21). Moreover, intravascular HA could interact with fibrinogen and accelerate thrombin-induced formation of fibrin clots (22,23). A recent study has demonstrated that exogenous HA can easily bound to human vascular endothelial cells of various organs including lung (24). Thus, we postulated that HA-associated dysregulation of hemostasis or vascular leakage might be a possible mechanism of HA-induced DAH. Particularly, as vagina wall is surrounded by extensive venous plexus (25), overdosing and procedure performed by nonmedical practitioner are thought to critical factors for DAH in our case. In addition, considering that HA is actively metabolized by hepatic clearance (2), hepatic dysfunction in our patient could be another possible contributing factor for the development of DAH. More investigations are required to elucidate how HA is involved in alveolar hemorrhage, whether HA interacts with alveolar-capillary interface, and what risk factors are associated with pulmonary complications of this agent.
In summary, we describe a case of DAH induced by illegal vaginal HA filler injection. While dermal and intra-articular injections of HA have proven to be a safe and effective treatment for cosmetics and pain relief, HA injection should be performed with caution. Clinician should recognize that HA, a most widely-using injectable agent, can be a cause of potentially fatal pulmonary complications.
Acknowledgements
Funding: This work was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (grant No. NRF-2017R1C1B5016828).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This manuscript was approved by the Institutional Review Board of Kyung Hee University Hospital (approval No. KH2018-06-044). Written informed consent was obtained from the patient.
References
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem 2002;277:4581-4. [Crossref] [PubMed]
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997;242:27-33. [Crossref] [PubMed]
- Wang A, de la Motte C, Lauer M, et al. Hyaluronan matrices in pathobiological processes. FEBS J 2011;278:1412-8. [Crossref] [PubMed]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev 2011;91:221-64. [Crossref] [PubMed]
- Greene JJ, Sidle DM. The Hyaluronic Acid Fillers: Current Understanding of the Tissue Device Interface. Facial Plast Surg Clin North Am 2015;23:423-32. [Crossref] [PubMed]
- Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg 2009;25:86-94. [Crossref] [PubMed]
- Baumann L. Dermal fillers. J Cosmet Dermatol 2004;3:249-50. [Crossref] [PubMed]
- Hoffmann K. Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: prospective European study. BMC Dermatol 2009;9:9. [Crossref] [PubMed]
- Park HJ, Jung KH, Kim SY, et al. Hyaluronic acid pulmonary embolism: a critical consequence of an illegal cosmetic vaginal procedure. Thorax 2010;65:360-1. [Crossref] [PubMed]
- Famularo G, Liberati C, Sebastiani GD, et al. Pulmonary embolism after intra-articular injection of methylprednisolone and hyaluronate. Clin Exp Rheumatol 2001;19:355. [PubMed]
- Bhagat R, Forteza RM, Calcote CB, et al. Pulmonary emboli from therapeutic sodium hyaluronate. Respir Care 2012;57:1670-3. [Crossref] [PubMed]
- Jang JG, Hong KS, Choi EY. A case of nonthrombotic pulmonary embolism after facial injection of hyaluronic Acid in an illegal cosmetic procedure. Tuberc Respir Dis (Seoul) 2014;77:90-3. [Crossref] [PubMed]
- Basora JF, Fernandez R, Gonzalez M, et al. A case of diffuse alveolar hemorrhage associated with hyaluronic acid dermal fillers. Am J Case Rep 2014;15:199-202. [Crossref] [PubMed]
- Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 2010;137:1164-71. [Crossref] [PubMed]
- Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000;6:430-5. [Crossref] [PubMed]
- Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res 1971.25-32. [Crossref] [PubMed]
- Crafoord S, Stenkula S. Healon GV in posterior segment surgery. Acta Ophthalmol (Copenh) 1993;71:560-1. [Crossref] [PubMed]
- Richter AW, Ryde EM, Zetterstrom EO. Non-immunogenicity of a purified sodium hyaluronate preparation in man. Int Arch Allergy Appl Immunol 1979;59:45-8. [Crossref] [PubMed]
- Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg 2006;117:3S-34S; discussion 5S-43S.
- Anandagoda N, Ezra DG, Cheema U, et al. Hyaluronan hydration generates three-dimensional meso-scale structure in engineered collagen tissues. J R Soc Interface 2012;9:2680-7. [Crossref] [PubMed]
- Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007;143:155-63. [Crossref] [PubMed]
- Weigel PH, Frost SJ, LeBoeuf RD, et al. The specific interaction between fibrin(ogen) and hyaluronan: possible consequences in haemostasis, inflammation and wound healing. Ciba Found Symp 1989;143:248-61; discussion 261-4, 281-5.
- Frost SJ, Weigel PH. Binding of hyaluronic acid to mammalian fibrinogens. Biochim Biophys Acta 1990;1034:39-45. [Crossref] [PubMed]
- Szczepanek K, Kieda C, Cichy J. Differential binding of hyaluronan on the surface of tissue-specific endothelial cell lines. Acta Biochim Pol 2008;55:35-42. [PubMed]
- Mazloomdoost D, Westermann LB, Mutema G, et al. Histologic Anatomy of the Anterior Vagina and Urethra. Female Pelvic Med Reconstr Surg 2017;23:329-35. [Crossref] [PubMed]


