James Neil Gilliam, MD—the career arc of a patient-oriented translational clinical investigation changemaker in rheumatologic skin disease
The early days
Dr. Gilliam was born on November 30, 1936 in Goldthwaite, a small rural “railroad town” in central Texas. After his undergraduate pre-medical work at Baylor University and the University of Texas in Austin he attended Baylor University School of Medicine in Houston, Texas. During his third year of medical school he took a six-month rotation visiting the Rheumatology (Internal Medicine) Unit at the University of London at Westminster Hospital.
He began his internal medicine internship and residency training at the UT Southwestern Medical School and Parkland Memorial Hospital in Dallas, Texas. Dr. Gilliam then served as a physician in the US Air Force in 1966–1968 in the 852nd Medical Group, Castle Air Force Base California. He began his dermatology residency and dermatopathology fellowship training at Stanford University, completing both in 1970. He then returned to UT Southwestern where he completed fellowship training in Dr. Morris Ziff’s Rheumatology Division in 1973.
Dr. Gilliam took a faculty position as an Assistant Professor in the Dermatology Division of the Department of Internal Medicine at UT Southwestern starting in 1972. He would later serve as the Founding Chairman of the Department of Dermatology at UT Southwestern in 1982 (Figure 1).

Marriage and children
Dr. Gilliam was survived by his wife of 19 years, Anita C. Gilliam, MD, PhD (June 19, 1943–November 21, 2015) and two daughters, Lisa K. Gilliam, MD (November 21, 1968) and Amy E. Gilliam, MD (September 30, 1970). Following Dr. Gilliam’s death in 1984, each of these three women went on to complete undergraduate and postgraduate medical training. They were to become the ultimate testament to the supportive, encouraging influence that Dr. Gilliam had on those around him including family, friends, protégées and colleagues.
After obtaining a PhD degree in molecular biology, Dr. Anita Gilliam went on to complete medical school, dermatology residency and dermatopathology fellowship training. She made important contributions to our understanding of the molecular immunology of the scleroderma/systemic sclerosis tissue reaction during her academic career at Yale University and Case Western Reserve University. Unfortunately, her professional career was also abbreviated by cancer in 2015. Jim Gilliam’s older daughter, Lisa, completed training in internal medicine and endocrinology and his younger daughter, Amy, completed training in dermatology and pediatric dermatology.
Professional influences
Dr. Gilliam was influenced by earlier clinical investigators who had worked on cutaneous lupus erythematosus (LE) in that era. Those individuals included Denny L. Tuffanelli, John H. Epstein, William L. Epstein, and Kimie Fukuyama, all of whom were affiliated with the Department of Dermatology at the University of California at San Francisco. In addition, he appreciated the earlier work on cutaneous LE of Neville Rowell and colleagues at the University of Leeds. He also admired the work several contemporaries—William Weston at the University of Colorado and Tom Provost at the University at Buffalo/The State University of New York. The efforts of these two investigative groups resulted in the initial description of the neonatal LE/congenital heart block autoimmune phenomena and the recognition of “ANA-negative SLE” as an alternate persona of patients with subacute cutaneous LE. The common link between neonatal LE/congenital heart block and ANA-negative SLE was the presence of circulating Ro/SS-A and La/SS-B autoantibodies and the presence of the 8.1 ancestral HLA haplotype upon which these autoantibodies arise.
Having trained in rheumatology, Dr. Gilliam also admired the efforts of earlier rheumatologists whose work included a consideration of the cutaneous manifestations of LE. Those included Naomi F. Rothfield and Edward L. Dubois. The work of experimental rheumatologists who were applying basic immunologic assay technologies such as such Ouchterlony double immunodiffusion to the identification of new disease-related autoantibodies also greatly influenced Dr. Gilliam. Such individuals included Morris Reichlin and Eng Tan.
Ed Dubois was a practicing rheumatologist and clinical scholar affiliated with the University of Southern California. Dr. Gilliam especially admired Dr. Dubois’ work in systematically cataloging the clinical manifestations of large numbers of SLE patients including a careful elucidation of their cutaneous findings. Denny Tuffanelli assisted Ed Dubois in important aspects of that effort.
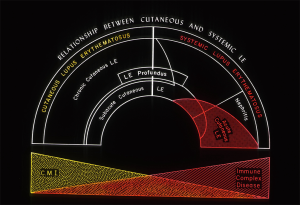
Ed Dubois was a proponent of the concept that the heterogeneous manifestations of a disorder such as LE could best be conceptualized as a spectrum of illness ranging from clinically mild manifestations in the skin and/or musculoskeletal system to fully expressed autoimmune injury of vital internal organs including the kidneys and central nervous system (1). This LE spectrum concept resonated with Dr. Gilliam. It was Dr. Gilliam’s adaptation and extension of Ed Dubois’ work in this area that gave birth to the concept that different skin manifestations of LE could reflect differing level of risk for associated internal organ damage from the systemic LE autoimmune process (Figure 2).
Alvin J. Cox Jr, with whom Dr. Gilliam trained in dermatopathology while at Stanford, influenced Dr. Gilliam’s thinking about the histopathologic similarities and differences between the various clinical cutaneous manifestations of LE. Dr. Gilliam was also strongly influenced by those who had been working on the new dermatologic immunology technique of immunofluorescence microscopy examination of lesion and non-lesional skin tissue as applied to clinical disorders such as cutaneous LE and cutaneous dermatomyositis. Dermatology leaders in that new clinical immunology field included Baart de la Faille-Kuyper, Ernest Beutner, and Tom Burnham.
These varied influences came together to provide Dr. Gilliam with a rather unique perspective on the LE spectrum of autoimmune illnesses. This outlook allowed him as a patient-oriented translational clinical investigator (POTCI) to make important contributions to our understanding of the complex relationships that exist between the cutaneous and systemic manifestations of LE.
Published works
Initial description of subacute cutaneous LE and presentation of a new classification of LE skin disease
In 1972, Dr. Gilliam brought his multidisciplinary medical training to bear in a joint faculty position in the Dermatology and Rheumatology Divisions at UT Southwestern Medical School in Dallas, Texas. His early research work under the guidance of Morris Ziff, widely recognized as the father of modern American Rheumatology, was aided by new and evolving laboratory technology that could be applied to such questions including the immunofluorescence microscopy examination of skin biopsy material and autoimmune serologic analysis of blood samples.
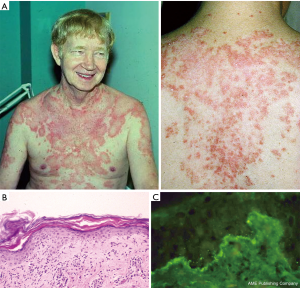
During his early work at UT Southwestern Dr. Gilliam encountered Caucasian patients of Northern European ancestry who had a widespread, photosensitive, nonscarring, often-annular dermatosis that displayed an inflammatory interface dermatitis reaction pattern on skin biopsy. Such patients often complained of joint aches, fatigue and loss of energy suggesting the presence of an underlying systemic inflammatory disease process. These patients typically had a positive antinuclear antibody assay and a continuous, granular, band-like array of immunoglobulin and complement components at the dermal-epidermal junction of lesional skin biopsies by direct immunofluorescence microscopy exam (i.e., lesional “lupus band”). These clinical and laboratory features differed significantly from those of patients presenting with classical scarring discoid LE skin lesions. Dr. Gilliam’s early work with such patients cannot be attributed to selection bias as the patient population of Parkland Memorial Hospital in Dallas was equivalently made up of African-American, Hispanic, and Caucasian patients.
In Dr. Gilliam’s mind the above outlined clinical, histopathological and laboratory features strongly linked this clinical skin disorder to the LE autoimmune process. However, such patients had not previously been recognized as having a form of cutaneous LE. Over the previous two centuries, this cutaneous entity had been referred to by others under many different designations (lupus marginatus, symmetrical erythema centrifugum, disseminated discoid LE, autoimmune annular erythema, lupus gyrates repens). Dr. Gilliam first introduced this newly-recognized form of cutaneous LE under the designation “subacute cutaneous LE” in 1977 (2). In 1979, he and two younger trainees, Rick Sontheimer and J. Ray Thomas, formally reported the clinical, pathologic and immunologic aspects of their first 27 subacute cutaneous LE patients (3) (Figure 3).
The consensus classification of cutaneous LE in the USA at that time that had been previously formulated by O’Leary and Montgomery at the Mayo Clinic in Rochester. The O’Leary-Montgomery classification had maintained the traditional bundling of non-scarring subacute cutaneous LE patients together with individuals having generalized forms of scarring discoid LE in their “disseminate LE” category. Prior to the autoimmune serologic era of LE, such distinctions seemed to be of little clinical consequence.
The concept of subacute cutaneous LE would likely not have been widely accepted had it not been for the collaborative studies between Drs. Gilliam and Morris Reichlin at the University at Buffalo concerning possible autoantibody associations of subacute cutaneous LE. This collaborative work demonstrated that the subacute cutaneous LE clinical-pathologic phenotype was associated with serologic and HLA markers—Ro/SS-A and La/SS-B autoantibodies and the 8.1 ancestral HLA haplotype (4,5).
In Dr. Gilliam’s original formulation of the foundation of his new classification scheme for LE skin diseases in 1977, he subcategorized LE skin lesions into two groups—those that targeted epidermal and upper dermal tissue and those that targeted deep dermal and subcutaneous tissue (2). At this time there was no understanding of the immunopathologic mechanisms that might underlie and link together these different histopathologic patterns of LE skin inflammation. It is now recognized that dysregulated class I interferon expression followed by its downstream pro-inflammatory consequences including cytotoxic T cell injury resulting in the “interface dermatitis” pattern of autoimmune skin injury serves as the common link between the different clinical forms of LE specific skin disease.
Dr. Gilliam and colleagues presented a reformulation of his original classification statement in 1981 (6). This is the classification scheme that would later become known as the “Gilliam classification” of LE skin disease. This new classification framework focused on those skin lesions whose pathology included a lichenoid tissue reaction/interface dermatitis. The rationale for this reformulation was that acute cutaneous LE, subacute cutaneous LE, and chronic cutaneous LE (the Hallmark clinical subtype being classical discoid LE), were the skin lesions thought to be the most characteristic of the LE autoimmune process as well as being the most common clinical forms of LE skin disease. In addition, the presence of each of the three clinical forms of LE-specific skin disease appeared to reflect different levels of risk for a patient’s developing clinically-significant systemic LE disease activity and damage to vital internal target organs.
Dr. Gilliam’s new classification systemic introduced the concept of “LE-nonspecific skin disease.” This was his designation for a large group of histopathologically-diverse inflammatory skin disorders that share a clinical association with the LE autoimmunity process but are not in themselves characteristic of that process. The “LE-nonspecific” designation indicated that such skin lesions are seen more frequently in patients known to have systemic LE activity and damage but could also be seen in other clinical disorders that were unrelated to LE. Examples include cutaneous small-vessel leukocytoclastic vasculitis (expressed clinically as either palpable purpura or urticarial vasculitis), bullous systemic LE and the cutaneous manifestations of the antiphospholipid antibody syndrome including livedo reticularis/livedo racemosa. While such lesions can be encountered in association with systemic LE, the same clinical and histopathologic manifestations can be seen in other clinical settings (e.g., palpable purpura of mixed cryoglobulinemia secondary to hepatitis C virus infection, drug hypersensitivity reactions, etc.).
The Gilliam classification of LE skin disease remains in widespread use today by dermatologists and rheumatologists internationally following its original formulation in 1981. And the clinical concept of “subacute cutaneous LE” is now a standard element in the modern language of LE. On December 30, 2017, a Google Scholar search using the phrase “subacute cutaneous lupus erythematosus” yielded 43,700 hits.
Subsequent evolution of thought concerning the subacute cutaneous LE concept
Following Dr. Gilliam’s death in 1984, his research interests were continued under the direction of one of his protégés, Rick Sontheimer, the author of this changemaker POTCI biography. In addition, over the next 5 years other workers in the field in the USA and Europe continued to examine and build upon the clinical concept of subacute cutaneous LE. These clinical investigators included Thomas Provost, Lela Lee, Jeffrey Callen, David Norris, Clark Huff, Jau-Shyong Deng, Peter Kind, Annagret Kuhn, Thomas Ruzicka, Tsu-San Lieu, Fukumi Furukawa, and Carmen Herrero among others. By the turn of the millennium an international consensus had developed concerning subacute cutaneous LE skin lesions being a clinical marker for an immunogenetically-distinct subset of LE patients (7-12).
Subacute cutaneous LE being recognized as a mosaic of autoimmunity
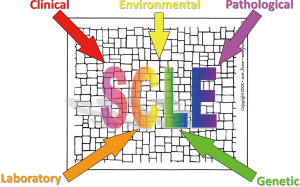
In 1989, Shoenfeld and Isenberg suggested that complex multisystem autoimmune disorders such as LE can be viewed as a “mosaic of autoimmunity” (13). When certain small disease “pieces” come together (genetic, environmental, hormonal, immunologic), a recognizable picture emerges. Subacute cutaneous LE has become a dermatologic example of the “mosaic of autoimmunity” (7).
Figure 4 provides a graphic illustration of this concept. When genetic predisposition [aberrant immune regulatory genes (i.e., the 8.1 ancestral haplotype)] and environmental stimuli (i.e., sunlight, infections) come together, autoimmunity results (i.e., Ro/SS-A autoantibody production). Humoral and cellular autoimmune responses in the skin then conspire to produce class I interferon-mediated tissue damage (i.e., interface dermatitis, lupus band development) producing clinically-characteristic photosensitive subacute cutaneous LE skin lesions.
Recognition and characterization of drug-induced subacute cutaneous LE
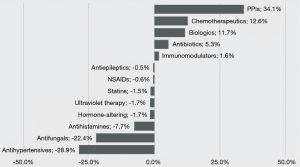
Reed and coworkers reported the first cases of drug-induced subacute cutaneous LE in 1985 caused by oral hydrochlorothiazide therapy (14). Since then a large number of different systemic drug classes have been reported as being chemical triggers for the initial clinical expression of subacute cutaneous LE skin lesions [(data reviewed in (15-18)] (Figure 5). The clinical and laboratory features of drug-induced subacute cutaneous LE have not varied significantly from those of idiopathic subacute cutaneous LE. However, the molecular and cellular mechanisms that are responsible for the initial precipitation of subacute cutaneous LE skin lesions in this drug induced clinical phenomenon remain unresolved. Precipitation of subacute cutaneous LE skin lesions in a predisposed host as a result of the isomorphic response of Koebner by drug-induced photosensitization may be one such mechanism.
Disease associations of subacute cutaneous LE
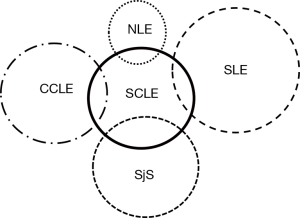
Subacute cutaneous LE can be seen to overlap with other rheumatologic skin conditions. Figure 6 displays a graphic illustration of such associations. Shortly after Dr. Gilliam’s death in 1984 it was realized that patients with subacute cutaneous LE are at risk for developing clinical features of Sjogren’s syndrome. This was not totally unexpected as these two clinical conditions are known to share the same immunogenetic predisposition—the production of Ro/SS-A and La/SS-B autoantibodies on the 8.1 ancestral haplotype genetic background. Subsequent study also identified a low level of overlap between subacute cutaneous LE and chronic cutaneous LE (especially classic discoid LE) as well as clinically-significant systemic LE (i.e., functional damage to vital internal target organs such as the kidneys and central nervous system). Also, women who have Ro/SS-A autoantibodies in their circulation who become pregnant are at risk for their pregnancies being complicated by neonatal LE (congenital heart block, characteristic skin lesions, hepatosplenomegaly and thrombocytopenia).
Molecular characterization of the Ro/SS-A autoantigen
Dr. Gilliam was intrigued by the molecular identity of the “cytoplasmic” ribonucleoproteins that formed precipitation lines in Ouchterlony doubled immunodiffusion with Ro/SS-A and La/SS-B autoantibodies present in subacute cutaneous LE patient serum. Unfortunately, his life was cut short before he could drill down experimentally into this problem himself. However, his dermatology coworkers at UT Southwestern including the author of this biography in collaboration with Dr. J. Donald Capra, MD, a Professor of Microbiology at UT Southwestern (https://en.wikipedia.org/wiki/J._Donald_Capra), were able to subsequently pursue this line of investigation.
This work led to the cloning and sequencing of the human form of calreticulin, a multifunctional, calcium-binding chaperone protein which at the time was thought to reside exclusively within the lumen of the endoplasmic reticulum. However, it was later shown to move between different intracellular compartments and appear at the cell surface and as a secreted form in the blood circulation (19,20). Calreticulin was initially thought to represent the major autoantigen-bearing 60 kDa polypeptide component of the Ro/SS-A ribonucleoprotein (19,21). However, following a period of controversy it was demonstrated that calreticulin was not the primary autoantigenic 60 kDa Ro/SS-A polypeptide (22,23). The basis for this controversy has been previously reviewed (24).
In addition to subacute cutaneous LE patients, calreticulin autoantibodies can be found in the serum of patients with Sjogren’s syndrome and systemic LE (25). Calreticulin autoantibodies have also been reported to occur in conjunction with Ro/SS-A autoantibodies in the blood of infants with complete congenital heart block (26). Calreticulin autoantibodies as detected by solid-phase immunoassay have also been reported to be present in the number of other unrelated conditions including inflammatory rheumatoid arthritis, mixed connective tissue disease (MCTD), celiac disease, primary biliary cirrhosis and parasitic infections. Therefore, calreticulin autoantibodies with lower binding affinity than those detected by immunoprecipitation appear not to be playing a specific pathogenetic role in subacute cutaneous LE or Sjogren’s syndrome. The role(s) of calreticulin autoantibodies in tissue injury patterns observed in these clinical disorders remains be determined.
Calreticulin has been reported to have a direct binding association with the 52 kDa Ro/SS-A polypeptide as well as the hYRNA nucleotide backbone of Ro/SS-A ribonucleoprotein particles (27,28). Calreticulin does appear to have a binding interaction with a subset of Ro/SS-A ribonucleoprotein particles that are devoid of the La/SS-B polypeptide (29). The spreading of immunity from the Ro52 and Ro60 polypeptides to calreticulin in experimental mice studies, suggesting that these three molecules have a physical association in some stage of cell biology (30). However, the lack of immunity spreading to the La/SS-B polypeptide observed in these immunized mice studies supports the possibility that calreticulin has a binding association with a subpopulation of Ro/SS-A particles that do not contain the La/SS-polypeptide.
In retrospect, the earlier controversy concerning calreticulin’s association with the autoantigenic Ro/SS-A ribonucleoprotein particle system can be viewed from several different perspectives.
Recent observations and opinion suggest that calreticulin is an unconventional, intrinsically-disordered protein. The biological functions of traditional proteins such as enzymes or transcription factors are defined by stable three-dimensional structures resulting from their amino acid sequences. However, it more recently has become clear that some proteins contain intrinsically disordered regions having a dynamic conformational structure resulting in changes between several metastable conformations in response to a variety of environmental stimuli (31).
The intrinsically-disordered nature of calreticulin was likely responsible for one of the early points of confusion concerning its association with the Ro/SS-A ribonucleoprotein particle system. Early immunoprecipitation studies with systemic LE and Sjogren’s syndrome patient sera followed by SDS-PAGE and Western blot analysis had confirmed that Ro/SS-A autoantigenic epitopes were present on a 60 kDa polypeptide. Work by the author and his colleagues at UT Southwestern following Dr. Gilliam’s death had identified calreticulin within the immunoprecipitate resulting from interaction of Ro/SS-A antibody containing the patient’s serum and Wil-2 cell extracts. This led to the assumption that calreticulin was likely to be the primary Ro/SS-A autoantigen-bearing polypeptide as calreticulin had an observed molecular weight of 60 kDa in SDS-PAGE and Western blot analysis. However, once human calreticulin was cloned its actual molecular weight was calculated to be 46 kDa based on its amino acid sequence. It is now thought that the intrinsically disordered nature of calreticulin is responsible for this SDS PAGE migration artifact (31).
The early controversy concerning calreticulin’s association with Ro/SS-A ribonucleoprotein particles could also relate to differences in immunoprecipitation techniques used by different groups of investigators. Dr. Gilliam’s coworkers employed Wil-2 cell extracts in their immunoprecipitation studies. The Wil-2 cell line consists of an Epstein-Barr virus transformed human lymphoblastoid B cells. Alspaugh and coworkers employed Wil-2 cell extracts in their immunoprecipitation studies that first identified SS-A and SS-B autoantibodies in Sjogren’s syndrome patients (32). Other groups of investigators studying the Ro/SS-A:La/SS-B autoantigen system in the 1980s and 1990s used human tissue extracts (e.g., spleen) or other transformed human cell lines (e.g., Hela cells) as autoantigenic extracts for their immunoprecipitation studies.
There is now growing evidence that Epstein-Barr virus infection plays a role in the etiopathogenesis and clinical course of systemic LE (33-36). In addition, there have been reports relating to a relationship between the Epstein-Barr virus infection and the Ro/SS-A autoantibody response in systemic LE and Sjogren’s syndrome patients (37-41).
Other endogenous human viruses such as cytomegalovirus have been reported to alter the cellular expression of calreticulin (42). In addition, calreticulin has been shown to be host cell protein that is known to interact with the rubella virus RNA (43,44). Calreticulin has also been reported to play a lectin-type chaperone role for several viral glycoproteins within the endoplasmic reticulum. More work is needed on the impact of Epstein-Barr virus infection on the cell biology of human calreticulin and the Ro/SS-A ribonucleoprotein particle system.
The cutaneous lupus band phenomenon and systemic LE disease activity
Other investigators employing the new immunofluorescence microscopy technology in the 1960s had described the presence of immunoglobulin and complement components deposited in a continuous, granular, band-like array at the dermal-epidermal junction of LE skin lesions (i.e., lesional lupus band) (Figure 3). Dr. Gilliam and his coworkers, Eric Hurd and Morris Ziff, were the first to report in 1974 the presence of a lupus band in normal-appearing non-lesional skin of patients with systemic LE (i.e., non-lesional lupus band). They observed that when present in sunlight-protected skin sites this non-lesional cutaneous immunologic phenomenon correlated positively with the presence of lupus nephritis (45). This phenomenon become known as the “lupus band test”. However, it is now thought that this immunopathologic phenomenon does not provide more clinical information beyond that of a reliable assay for double-stranded DNA autoantibodies in the blood which is less invasive and less costly to perform.
Dr. Gilliam had an interest in examining the cutaneous parameters of murine models of systemic LE autoimmunity including the NZB/NZW F1 hybrid mouse. He and his coworkers were the first to identify the presence of a non-lesional lupus band in the skin of NZB/NZW F1 hybrid mice (46). And as in humans, they observed a positive correlation between the appearance of such non-lesional lupus bands and the onset of active nephritis in this experimental animal model.
Other observations pertaining to cutaneous and systemic LE
In addition to subacute cutaneous LE, Dr. Gilliam had a focus of interest on the epidemiology and clinical immunology of classical discoid LE. He and his colleagues enhanced our understanding of the epidemiology and clinical immunologic associations of African Americans whose disease presentation was dominated by classical discoid LE skin lesions (47).
Dr. Gilliam also became interested in the evolving concept of overlapping autoimmune connective tissue disorders, including MCTD. He made observations related to myocardial inflammation occurring in MCTD (48,49). And, he helped introduce the MCTD clinical concept to the dermatology community by reviewing the cutaneous features that have been reported to occur in such patients at that time.
In addition, Dr. Gilliam led an effort to further refine and validate a new indirect immunofluorescence assay for double-stranded DNA autoantibodies which are highly-specific for systemic LE (50-54). This assay was based upon the kinetoplast of Crithidia luciliae, a hemoflagellate parasite of the housefly. The C. luciliae intracellular kinetoplast contains double-stranded DNA in a circular configuration that avoids single-stranded DNA contamination. The double-stranded DNA autoantigen employed by other double-stranded DNA autoantibody assay technologies at that time were subject to being contaminated by single-stranded DNA making them less reliable for the detection of double-stranded DNA autoantibodies that are an important parameter for the diagnosis of systemic LE. Thus, the technically simpler and more specific C. luciliae double-stranded DNA antibody assay rapidly became the gold standard for double-stranded DNA autoantibody confirmation in clinical laboratories. This assay remains in use today for this purpose.
Discovery of a new therapeutic strategy for livedoid vasculopathy
Idiopathic livedoid vasculopathy (Syn primary atrophie blanche, livedoid vasculitis) is a rare, chronically-recurring condition that results in the development of exquisitely-painful ulcers in the skin of the lower legs, ankles and feet (Figure 7). In the mid-1970s, the cause(s) of this condition was unknown. And, the condition was extremely difficult to treat empirically. Because of the severe chronic pain it produced, patients often ended up addicted to opioid narcotics.
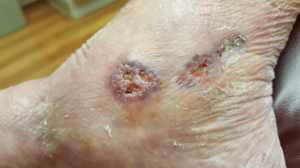
Dr. Gilliam and colleagues observed that the histopathologic findings of the primary lesions of this condition on skin biopsy suggested that it might be the result of a thrombotic micro-vasculopathy of the skin. He and his colleagues postulated that fibrinolytic medical therapy might be of benefit for this condition. Work by others in that era had indicated that the combination of two available drugs at that time, phenformin and ethylestrenol, enhanced fibrinolysis in animal models. Taking this lead, Dr. Gilliam and his colleagues began to treat livedoid vasculopathy patients with the combination of phenformin and ethylestrenol in an uncontrolled clinical fashion with spectacular clinical results (55). Because of side effects, both phenformin (lactic acidosis) and ethylestrenol (hepatotoxicity) were subsequently withdrawn from the USA market. Sadly, Dr. Gilliam’s livedoid vasculopathy patients that had been in complete, pain-free remission on this combination therapy were observed to slowly remit after these drugs were no longer available in the USA. Interestingly, much of the more recent clinical work in this area has focused on more modern therapeutic approaches to anticoagulation and fibrinolysis.
Contribution to the initial immunologic characterization of epidermal Langerhans cells
In 1980, Paul Bergstresser, Galen Toews and Wayne Streilein at UT Southwestern were among the first to report that murine epidermal dendritic Langerhans cells had unique immunobiological characteristics (56). Dr. Gilliam later joined this team in further studies of the immunobiology of these cells (57,58).
Identification of the immunogenetic associations of herpes gestationis
Herpes gestationis is a rare, autoimmune, vesiculobullous disease of pregnancy or the immediate post-partum period that is characterized by the deposition of complement (and occasionally immunoglobulin) within the lamina lucida of the cutaneous basement membrane zone. In addition to examining the epidemiology of this rare autoimmune disorder in the early 1980s, Dr. Gilliam and his colleagues Jeffrey Shornick and Peter Stastny, were the first to report that this condition had a genetic association with HLA-DR3 and DR4 (59-62).
Untimely death and legacy
Dr. Gilliam died tragically on June 6, 1984 at the age of 52 years after a closely-held, courageous five-year battle with an aggressive form of prostate cancer. It was only 2 years earlier that he had been named the Founding Chairman of the newly-designated Department of Dermatology at UT Southwestern Medical Center in Dallas.
Dr. Gilliam was a large, gregarious man and a natural-born leader. Those who were close to him endearingly referred to him as “The Bear.” And, like a mother bear he was fiercely protective and supportive of the younger people within his spheres of influence. As an example, Dr. Gilliam selflessly chose to spend the last good year of his life preparing an NIH research grant renewal application. In retrospect, it became clear that this effort was largely for the benefit of his younger research colleagues who would be left behind after his death. Not surprisingly, these personality traits engendered extreme loyalty and affection among those with whom he worked (Figure 8).

Dr. Gilliam was generously endowed with mentoring talent. The diverse, unproven group of trainees and colleagues with whom he chose to surround himself at UT Southwestern in the 1970s was emboldened and empowered by this mentoring talent. A disproportionate number of those individuals went on to long-term clinically-oriented research and academic leadership positions in American dermatology, dermatopathology and rheumatology.
Dr. Gilliam will be remembered professionally as a successful POTCI and academic leader. But perhaps the most enduring memory will be the impact as a Mentor’s Mentor and true friend that he had on the people around him. This aspect of his legacy is summed up by a passage from Dr. Paul Bergstresser’s memorial to Dr. Gilliam that was published following his death, “The tragedy of Gilliam’s unsuccessful fight with cancer lies not in his death, which had become inevitable, but in the fact that he and his friends had been given so little time together. For it was in working with others that the genius of Jim Gilliam emerged.” (63).
Honors, awards
- US Strategic Air Command Commendation for “Highest professional standards, selfless devotion to duty and personal concern for the welfare of those served”, 1968;
- Veterans Administration Clinical Investigator Award, 1975;
- US Public Health Service Research Career Development Award, 1977–1982;
- Dermatology Foundation, Frederick G. Novy, Jr. Memorial Lectureship, 1978;
- Election to American Society for Clinical Investigation, 1980;
- Election to American Dermatological Association, 1981;
- The American Lupus Society Award “In recognition of leadership in the field of lupus research”, 1982;
- James N. Gilliam, MD. Endowed Chair in Dermatology. Established in the Department of Dermatology at UT Southwestern Medical Center in Dallas in 1997.
External links
Published memorials
- https://ac.els-cdn.com/S0022202X15435158/1-s2.0-S0022202X15435158-main.pdf?_tid=9297bf76-ecf1-11e7-8960-00000aab0f6c&acdnat=1514590975_0b48112bc4a584b5f6a45ba8fac8109c (63);
- http://www.cidjournal.com/article/0738-081X(88)90043-0/abstract (64);
- https://books.google.com/books?id=vsCbHAAACAAJ&dq=bibliogroup:%22The+journal+of+investigative+dermatology%22&hl=en&sa=X&ved=0ahUKEwjlzfj-9LHYAhUY1WMKHYSZD2oQ6AEIKTAA.
Others links of interest
- https://www.sciencedirect.com/science/article/pii/S0022202X15435158;
- http://www.utsouthwestern.edu/education/medical-school/departments/dermatology/faculty.html;
- http://www.utsouthwestern.edu/education/medical-school/departments/dermatology/;
- https://www.the-dermatologist.com/content/spotlight-amy-e-gilliam-md.
Summary
In summary, it can be argued that Dr. James Neil Gilliam fulfilled the criteria as a changemaker POTCI in his area of interest in autoimmune skin disorders. His multidisciplinary medical training in internal medicine, dermatopathology, dermatology and rheumatology endowed him with the global perspective to recognize that a number of poorly-understood inflammatory skin disorders might yield to a better understanding of the cellular and molecular expression of autoimmune responses in human skin. And, he adopted the new tools of basic immunologic research for a better understand of the pathophysiology of cutaneous LE and other inflammatory skin disorders.
It has been said that “A mentor is someone who sees more talent and ability within you, than you see in yourself, and helps bring it out of you.” [Bob Proctor, modern American author and motivational speaker (https://everipedia.org/wiki/bob-proctor/)]. Dr. James N. Gilliam’s innate talents as a team leader and Mentor served to magnify the impact of his abbreviated clinical research career in a manner that continues to reverberate even today in those with whom he had worked. Dr. Gilliam was always at his best when he was among people where his catalytic interpersonal nature could shine its brightest (Figure 9).

Acknowledgements
Funding: Dr. Sontheimer’s research career has been supported by a Dermatology Foundation Fellowship, a National Institutes of Health (NIH) Clinical Investigator Award, a NIH Research Career Development Award and a NIH R01 Research Grant, “Mechanisms of Cutaneous Injury in Lupus Erythematosus” (AR19101).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Dubois EL, Tuffanelli DL. Clinical manifestations of systemic lupus erythematosus. JAMA 1964;190:104-11. [Crossref] [PubMed]
- Gilliam JN. The cutaneous signs of lupus erythematosus. Continuing Education for the Family Physician 1977;6:34-70.
- Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol 1979;115:1409-15. [Crossref] [PubMed]
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med 1982;97:664-71. [Crossref] [PubMed]
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest 1981;67:312-6. [Crossref] [PubMed]
- Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471-5. [Crossref] [PubMed]
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Auto immune Rev 2005;4:253-63.
- Parodi A, Caproni M, Cardinali C, et al. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. A review by the Italian group of immunodermatology. Dermatology 2000;200:6-10. [Crossref] [PubMed]
- Versapuech J, Beylot-Barry M, Doutre MS, et al. Subacute cutaneous lupus. Evolutive and therapeutic features of a series of 24 cases. Presse Med 2000;29:1596-9. [PubMed]
- Chlebus E, Wolska H, Blaszczyk M, et al. Subacute cutaneous lupus erythematosus versus systemic lupus erythematosus - Diagnostic criteria and therpeutic options. J Am Acad Dermatol 1998;38:405-12. [Crossref] [PubMed]
- Fabbri P, Bernacchi E, Neri R. Subacute cutaneous lupus erythematosus. Review of the literature and immunological studies of 11 patients. G Ital Dermatol Venereol 1990;125:329-36. [PubMed]
- Sontheimer RD. Subacute cutaneous lupus erythematosus: a decade's perspective. Med Clin North Am 1989;73:1073-90. [Crossref] [PubMed]
- Shoenfeld Y, Isenberg DA. The mosaic of autoimmunity. Immunol Today 1989;10:123-6. [Crossref] [PubMed]
- Reed BR, Huff JC, Jones SK, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med 1985;103:49-51. [Crossref] [PubMed]
- Lowe G, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2011;164:465-72. [PubMed]
- Callen JP. Drug-induced subacute cutaneous lupus erythematosus. Lupus 2010;19:1107-11. [Crossref] [PubMed]
- Gronhagen CM, Fored CM, Linder M, et al. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol 2012;167:296-305. [Crossref] [PubMed]
- Michaelis TC, Sontheimer RD, Lowe GC. An update in drug-induced subacute cutaneous lupus erythematosus. Dermatol Online J 2017.23. [PubMed]
- McCauliffe DP, Zappi E, Lieu TS, et al. A human Ro/SS-A autoantigen is the homologue of calreticulin and is highly homologous with onchocercal RAL-1 antigen and an aplysia "memory molecule". J Clin Invest 1990;86:332-5. [Crossref] [PubMed]
- Sontheimer RD, Nguyen TQ, Cheng ST, et al. The unveiling of calreticulin - A clinically relevant tour of modern cell biology. J Investig Med 1995;43:362-70. [PubMed]
- McCauliffe DP, Lux FA, Lieu TS, et al. Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. J Clin Invest 1990;85:1379-91. [Crossref] [PubMed]
- Rokeach LA, Haselby JA, Meilof JF, et al. Characterization of the autoantigen calreticulin. J Immunol 1991;147:3031-9. [PubMed]
- Ben-Chetrit E. The molecular basis of the SSA/Ro antigens and the clinical significance of their autoantibodies. Br J Rheumatol 1993;32:396-402. [Crossref] [PubMed]
- Sontheimer RD, Racila D, Racila E, et al. Calreticulin's role(s) in autoimmune disorders. In: Michalek M, Eggleton P. editors. Calreticulin. Second ed. Molecular Intelligence Series. New York: Landes Bioscience & Kluwer Academic Press, 2003:180-92.
- Sontheimer RD, Nguyen TQ, Cheng ST, et al. Calreticulin and autoimmunity. In: Michalak M. editor. Calreticulin. Austin: R.G. Landers Co., Biomedical Publishers, 1996:117-39.
- Orth T, Dörner T, Zum Buschenfelde KHM, et al. Complete congenital heart block is associated with increased autoantibody titers against calreticulin. Eur J Clin Invest 1996;26:205-15. [Crossref] [PubMed]
- Cheng ST, Nguyen TQ, Yang YS, et al. Calreticulin binds hYRNA and the 52 KD polypeptide component of the Ro/SS-A ribonucleoprotein autoantigen. J Immunol 1996;156:4484-91. [PubMed]
- Cheng ST, Nguyen TQ, Capra JD, et al. Recombinant calreticulin binds specifically to synthetic hYRNA. J Invest Dermatol 1995;104:653.
- Lieu TS, Sontheimer RD. A subpopulation of Wil-2 Cell calreticulin molecules is associated with Ro/SS-A ribonucleoprotein particles. Lupus 1997;6:40-7. [Crossref] [PubMed]
- Kinoshita G, Keech CL, Sontheimer RD, et al. Spreading of the immune response from 52kDa Ro and 60kDa Ro to calreticulin in experimental autoimmunity. Lupus 1998;7:7-11. [Crossref] [PubMed]
- Varricchio L, Falchi M, Dall'Ora M, et al. Calreticulin: Challenges Posed by the Intrinsically Disordered Nature of Calreticulin to the Study of Its Function. Front Cell Dev Biol 2017;5:96. [Crossref] [PubMed]
- Alspaugh MA, Tan EM. Antibodies to cellular antigens in Sjogren's syndrome. J Clin Invest 1975;55:1067-73. [Crossref] [PubMed]
- Harley JB, Chen X, Pujato M, et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat Genet 2018;50:699-707. [Crossref] [PubMed]
- James JA, Kaufman KM, Farris AD, et al. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest 1997;100:3019-26. [Crossref] [PubMed]
- Han L, Zhang Y, Wang Q, et al. Epstein-Barr virus infection and type I interferon signature in patients with systemic lupus erythematosus. Lupus 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Chougule D, Nadkar M, Rajadhyaksha A, et al. Association of clinical and serological parameters of systemic lupus erythematosus patients with Epstein-Barr virus antibody profile. J Med Virol 2018;90:559-63. [Crossref] [PubMed]
- Agmon-Levin N, Dagan A, Peri Y, et al. The interaction between anti-Ro/SSA and anti-La/SSB autoantibodies and anti-infectious antibodies in a wide spectrum of auto-immune diseases: another angle of the autoimmune mosaic. Clin Exp Rheumatol 2017;35:929-35. [PubMed]
- Cuomo L, Cirone M, Di Gregorio AO, et al. Elevated antinuclear antibodies and altered anti-Epstein-Barr virus immune responses. Virus Res 2015;195:95-9. [Crossref] [PubMed]
- Croia C, Astorri E, Murray-Brown W, et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjogren's syndrome. Arthritis Rheumatol 2014;66:2545-57. [Crossref] [PubMed]
- Kivity S, Arango MT, Ehrenfeld M, et al. Infection and autoimmunity in Sjogren's syndrome: a clinical study and comprehensive review. J Autoimmun 2014;51:17-22. [Crossref] [PubMed]
- Pasoto SG, Natalino RR, Chakkour HP, et al. EBV reactivation serological profile in primary Sjogren's syndrome: an underlying trigger of active articular involvement? Rheumatol Int 2013;33:1149-57. [Crossref] [PubMed]
- Zhu J, Newkirk MM. Viral induction of the human autoantigen calreticulin. Clinical and Investigative Medicine 1994;17:196-205. [PubMed]
- Singh NK, Atreya CD, Nakhasi HL. Identification of calreticulin as a rubella virus RNA binding protein. Proc Natl Acad Sci U S A 1994;91:12770-4. [Crossref] [PubMed]
- Nakhasi HL, Singh NK, Pogue GP, et al. Identification and characterization of host factor interactions with cis-acting elements of rubella virus RNA. Arch Virol Suppl 1994;9:255-67. [PubMed]
- Gilliam JN, Cheatum DE, Hurd ER, et al. Immunoglobulin in clinically uninvolved skin in systemic lupus erythematosus: association with renal disease. J Clin Invest 1974;53:1434-40. [Crossref] [PubMed]
- Gilliam JN, Hurd ER, Ziff M. Subepidermal deposition of immunoglobulin in NZB/NZW F hybrid mice. J Immunol 1975;114:133-7. [PubMed]
- Prystowsky SD, Herndon JH, Gilliam JN. Chronic cutaneous lupus erythematosus (DLE): A clinical and laboratory investigation of 80 patients. Medicine 1976;55:183-91. [Crossref] [PubMed]
- Gilliam JN, Prystowsky SD. Mixed connective tissue disease syndrome. Arch Dermatol 1977;113:583-7. [Crossref] [PubMed]
- Whitlow PL, Gilliam JN, Chubick A, et al. Myocarditis in mixed connective tissue disease. Association of myocarditis with antibody to nuclear ribonucleoprotein. Arthritis Rheum 1980;23:808-15. [Crossref] [PubMed]
- Deng JS, Rubin RL, Lipscomb MF, et al. Reappraisal of the specificity of the Crithidia luciliae assay for nDNA antibodies: evidence for histone antibody kinetoplast binding. Am J Clin Pathol 1984;82:448-52. [Crossref] [PubMed]
- Steinmetz SE, Deng JS, Rubin RL, et al. Reevaluation of specificity of Crithidia luciliae kinetoplast as a substrate for detecting antibodies to double-stranded deoxyribonucleic acid. J Am Acad Dermatol 1984;11:490-3. [Crossref] [PubMed]
- Sontheimer RD, Gilliam JN. A reappraisal of the relationship between subepidermal immunoglobulin deposits and DNA antibodies in systemic lupus erythematosus: a study using the Crithidia luciliae immunofluorescence anti-DNA assay. J Invest Dermatol 1979;72:29-32. [Crossref] [PubMed]
- Chubick A, Sontheimer RD, Gilliam JN, et al. An appraisal of tests for native DNA antibodies in connective tissue diseases. Clinical usefulness of Crithidia luciliae assay. Ann Intern Med 1978;89:186-92. [Crossref] [PubMed]
- Sontheimer RD, Gilliam JN. An immunofluorescence assay for double-stranded DNA antibodies using the Crithidia luciliae kinetoplast as a double-stranded DNA substrate. J Lab Clin Med 1978;91:550-8. [PubMed]
- Gilliam JN, Herndon JH, Prystowsky SD. Fibrinolytic therapy for vasculitis of atrophie blanche. Arch Dermatol 1974;109:664-7. [Crossref] [PubMed]
- Toews GB, Bergstresser PR, Streilein JW. Langerhans cells: sentinels of skin associated lymphoid tissue. J Invest Dermatol 1980;75:78-82. [Crossref] [PubMed]
- Bergstresser PR, Toews GB, Gilliam JN, et al. Unusual numbers and distribution of Langerhans cells in skin with unique immunologic properties. J Invest Dermatol 1980;74:312-4. [Crossref] [PubMed]
- Streilein JW, Toews GT, Gilliam JN, et al. Tolerance or hypersensitivity to 2,4-dinitro-1-fluorobenzene: the role of Langerhans cell density within epidermis. J Invest Dermatol 1980;74:319-22. [Crossref] [PubMed]
- Shornick JK, Meek TJ, Nesbitt LT Jr, et al. Herpes gestationis in blacks. Arch Dermatol 1984;120:511-3. [Crossref] [PubMed]
- Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-9. [Crossref] [PubMed]
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol 1983;8:214-24. [Crossref] [PubMed]
- Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens HLA-DR3 and DR4 in herpes gestations. J Clin Invest 1981;68:553-5. [Crossref] [PubMed]
- Bergstresser PR. In Memorium. J Invest Dermatol 1984;83:241. [Crossref]
- Sontheimer RD. Festschrift to Professor James N. Gilliam. Clin Dermatol 1988;6:1-2. [Crossref] [PubMed]


