Electrical impedance tomography
Introduction
Continuous assessment of respiratory status is one of the cornerstones of modern intensive care unit (ICU) monitoring systems. Lung imaging techniques have experienced overwhelming progress: we currently not only base our knowledge on chest X-ray or computed tomography, but use lung ultrasound, positron emission tomography, electrical impedance tomography (EIT) or magnetic resonance imaging to improve our understanding of the disease and the effect of therapeutic strategies (1-3).
EIT uses electrical currents to assess the conductivity distribution within the body from voltage measurements of its surface. This concept was first described as a method to explore subterranean mineral deposits in the early twentieth century. Medical use of EIT was developed in the 1980s by Barber and Brown at the Department of Medical Physics and Clinical Engineering in Sheffield (UK). A broad spectrum of possible applications in medicine were suggested, from gastric emptying to brain function monitoring, and then from breast imaging to interpretation of lung function. Since the first publication of an EIT image of the human thorax in 1985 by Brown et al., multiple advances have seen the light in this field of study, driven by the clinical need for monitoring of lung ventilation and assessment of regional lung function at the bedside (4). Several commercial EIT devices are currently available and being used on a day-to-day basis in different ICUs around the world: Pulmovista® 500 (Dräger Medical GmbH, Lündbeck, Germany), Goe-MF® II (CareFusion - Becton, Dickinson and Company, San Diego, United States), Mark 1® and Mark 3.5® (Maltron International Ltd, Rayleigh, United Kingdom), BB® (Swisstom AG, Landquart, Switzerland) and Enlight® (Timpel S/A, Sao Paulo, Brazil) (5).
Principles of EIT
EIT is a non-invasive, radiation-free, real-time imaging of ventilation. Regional electrical bioimpedance measurements require an electrode belt of 16 electrodes (or 32 in the current models by Swisstom AG and Timpel S/A manufacturers) placed around the chest wall, including a reference electrode connected to a central body, preferably to the abdomen (the reference electrode ensures that all measurements of the different electrode pairs are related to the same electrical potential). The 16-electrode belt is placed in one transverse plane, usually between the 4th and 5th intercostal space (it is not recommended to place the belt lower than the 6th intercostal space because the diaphragm may enter the measurement plane).
EIT images are determined by the distribution of intrathoracic bioimpedance. Bioimpedance is defined by the specific composition of tissues, taking into account water, electrolytes, fat, etc. High content of extracellular water, high concentration of electrolytes, large cells and a high number of cell connections reduce impedance, whilst fat accumulation, bone and air increase impedance. Therefore, pathological changes of the tissue composition (such as pleural effusion, lung fibrosis, alveolar fluid, etc.) entail a change in regional bioimpedance. Lung imaging is therefore an ideal application of EIT; large changes in lung volume (and therefore in conductivity) can be easily detected due to the closeness of the lungs to the surface (6-12).
Small alternating high frequency and low amplitude electrical currents are applied though a pair of electrodes while the resulting surface potentials (or voltages) are measured by the remaining electrodes. Applying Ohm’s law, the bioelectrical impedance between the injection and measurement electrode pairs is established from the known applied current and the measured voltages. Subsequently, the adjacent electrode pair is used for the next current application, and the other 13 measurements of voltage are carried out. The current applications and voltage measurements through adjacent electrode pairs imply a complete rotation around the thorax, creating voltage profiles at the 16 electrode positions, each composed of 13 voltage measurements. The resulting 208 values, also called frames (or raw images), are used to reconstruct a transverse EIT image; current EIT devices offer scan rates (number of frames acquired per second) of about 50 images per second.
In humans, an inspiratory maneuver from residual volume to total lung capacity amplifies the regional bioimpedance by approximately 300% (13). Cardiac activity and perfusion cause a change in thoracic bioimpedance, from diastole to systole, in a range of 3% (14). Extravascular lung water, body movement and electrode resistance in the skin may also change thoracic bioimpedance. In summary, an increase in intrapulmonary gas volume decreases conductivity, while an increase in blood or fluid volume, or disruption of cellular barriers, increase conductivity.
To be able to provide a high-resolution image, EIT requires a complex system of processors and software that compiles and analyzes all the information given by the electrodes and compares the recollected data with interindividual variations, adjusting to the thoracic geometry. A reconstruction algorithm will reject measurements at poorly connected electrodes, or ignore redundancy of data; they implicitly make assumptions about the level of random noise or interference in the raw data, and vary depending on the manufacturer: Sheffield back-protection (used by Goe-MF II and Mark 1 and 3.5), FEM-based Newton-Raphson method (used by PulmoVista and Enlight) and GREIT (used by BB).
EIT devices use the relative approach and thus calculate difference impedance images in relation to a reference. EIT images are usually represented on a pixel grid, where all image elements are the same size. Image reconstruction calculate images of change in tissue properties between a baseline measurement (reference) and the current frame, expressed as a percentage; these images are then represented by colour coding, which depends on the choice of the baseline frame (not unified between manufacturers). An EIT waveform is then defined as a sequence of impedance change values over time, represented in different ROI (or regions of interest), where image pixels are chosen to reflect regional changes associated with relevant effects (regions where ventilation related impedance changes occur).
Hence, obtaining functional EIT, we are able to visualize global and local ventilation activity over a period of time (selected by the user), picturing a map of ventilation distribution. Functional EIT produces EIT measures, defined as values derived from an EIT data acquisition. There are average global and regional measures (for example: regional or global ventilation delay or tidal variation), measures of spatial distribution of ventilation (for example, anterior/posterior ventilation ratio, center of ventilation, global inhomogeneity (GI) index] and examination-specific measures (e.g., regional respiratory system compliance or regional intratidal gas distribution). However, we must point out an important drawback; only regions of the thorax that change their impedance over time will be represented by the EIT images.
Clinical applications in acute respiratory distress syndrome (ARDS)
ARDS is relatively frequent in the intensive care patient population. ARDS is characterized by diffuse alveolar damage, associated with an increase in alveolar and capillary permeability due to mechanisms of tension, stretching and shear between alveolar units, leading to accumulation of interstitial and alveolar edema. Release of inflammatory mediators causes bronchoconstriction, embolic phenomena, vasoconstriction of the pulmonary arterioles, and finally fibrosis of the lung parenchyma. This pathophysiologic phenomena leads, on one hand, to an increase in the overall weight of the lung (causing alveolar collapse in the dependent lung regions due to gravity) and on the other hand, triggers an inflammatory response that determines the presence of multiorgan failure (15-17).
Physicians have been looking for methods to optimize alveolar recruitment, maintaining an open lung and limiting pulmonary overdistension. Protocolled mechanical ventilation settings are not adapted to each patient’s individual needs, and adjusting mechanical ventilation parameters is still the main bone of contention in clinical practice. We must highlight that ARDS has heterogeneous properties. Therefore, overall parameters reflecting the condition of the lung as a whole are insufficient to adequately target protective ventilation (18-21). All the systems that are being used for routine lung monitoring—graphic charts and ventilator data, radiological or gasometric tests—have limitations: either they provide information without regional specificity, or they do not provide progressive and continuous data over time.
In search of better ways to monitor pulmonary functions, EIT plays the lead. Lung EIT can be a diagnostic and a guiding tool for an adequate optimization of mechanical ventilation in critically ill patients, especially on ARDS, where mechanical ventilation remains a challenge.
Guidance on mechanical ventilation settings (22-39)
“Protective” ventilatory strategies have been designed to try to optimize mechanical ventilation and minimize its potential damages. These strategies are mainly based on the application of low tidal volumes and maneuvers designed to increase the functional residual capacity, trying to reduce cyclic alveolar collapse and overdistension of the lung.
As mentioned above, global parameters such as pressure-volume curves or measurement of the respiratory system compliance do not reliably translate what is actually happening in the lung, especially regarding to regional distribution of the tidal volume administrated. EIT images of the regional distribution of ventilation and changes in lung volume in real time highlight the relationship between the dependent and non-dependent lung regions. This dynamic evaluation makes EIT a helpful tool to optimize ventilator parameters on an individualized basis, as EIT may assist in defining mechanical ventilation settings, assess distribution of tidal volume and of end-expiratory lung volume (EELV) and contribute to titrate positive end-expiratory pressure (PEEP)/tidal volume combinations.
Studies performed in the last decade (23-32) have demonstrated that EIT-guided ventilation results in improved respiratory mechanics, improved gas exchange and reduced histologic evidence of ventilator-induced lung injury in an animal model. Careful titration of PEEP following maximal lung recruitment effectively reverses existing alveolar collapse and prevents further alveolar closure. EIT-based algorithms that estimate recruitable alveolar collapse, avoiding hyperdistension, have been one of the most interesting topics over the last years. Lung EIT has been used to assess and quantify the global and regional changes in lung impedance at the end of exhalation, quantifying gains (recruitment) and losses (overdistention or de-recruitment), granting a more realistic evaluation of different ventilator modes or recruitment maneuvers, and helping in the identification of responders and non-responders to such maneuvers (Figure 1).

Costa et al. (33) demonstrated good correlation between EIT and CT estimates of lung collapse during decremental PEEP trials after a maximal lung recruitment maneuver. GI index has been used to describe the inhomogeneity of tidal volume distribution in a cross-sectional lung plane, restricting the analysis to one lung region. In 2010 Zhao et al. (35,36) demonstrated that by adding small changes of PEEP, the gradient of the GI value indicated the direction of beneficial PEEP alteration. This same parameter was used in Liu et al. (37), where regions with overdistension were defined as those that presented high air levels on end-expiration but no air coming in or out during tidal breathing.
Recently, Eronia et al. (38) studied the feasibility of personalized PEEP selection based on its efficacy in stabilizing the EELV increase induced by a recruitment maneuver, using EIT as a tool to monitor EELV changes (parallel to end-expiratory lung impedance changes). Their study protocol consisted in three consecutive steps, comparing PEEP titration using the ARDSnet guidelines and PEEP titration with EIT after applying a continuous positive airway pressure of 40 cmH2O for 40 s, measuring end-expiratory lung impedance variation (∆EELI) thirty seconds and 10 minutes after the recruitment maneuver; if ∆EELI decreased more than 10% of ∆EELIstart a new recruitment maneuver was performed, and PEEP increased by 2 cmH2O; and if ∆EELI decreased less than 10% of ∆EELIstart, PEEP was left unchanged. They demonstrated that bedside PEEP setting based on sustained recruitment following a recruitment maneuver as visualized by EIT was achievable in the majority of the patients, leading to application of higher PEEP values and allowing quantification of regional overdistension associated with PEEP increase (Figures 2,3).
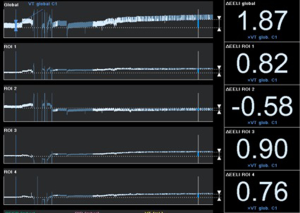
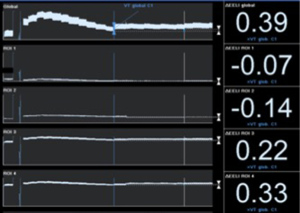
EIT has also been used to set PEEP for ECMO treated severe ARDS patients, providing real-time monitoring during ultra-protective ventilation with very low tidal volume. Franchineau et al. (39) emphasize the importance of preventing cyclic end-expiratory collapse, associated with increased ECMO blood flow, which might unnecessarily prolong ECMO duration and enhance the risk of ECMO-related complications. In the study they evaluate EIT ability to monitor a decremental PEEP trial (20 to 0 cmH2O) in a 5 cmH2O steps; EIT-based best compromise PEEP (defined in this case as the lowest pressure able to limit EIT-assessed collapse to under 15% with the least overdistension) was around 10 to 15 cmH2O, drawing attention to the broad variability in optimal PEEP observed in severe ARDS patients.
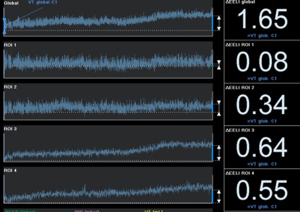
Lastly, high flow nasal cannula (HFNC) has been used in hypoxemic respiratory failure due to its physiological mechanisms (administration of high inspired oxygen fraction and creation of positive oropharyngeal airway pressure). HFNC under EIT monitoring has shown improved oxygenation by increasing both end-expiratory lung volume and tidal volume, regardless of body position suggesting an increase in functional residual capacity (40,41) (Figure 4).
So far, great progress has been made. However, even though EIT is becoming a more day-to-day tool regarding mechanical ventilation settings, we must remind that all parameters (severity of illness, hemodynamic tolerance, CT images, respiratory mechanics, …) must be taken into account when titrating PEEP. According to (42) the multicenter, prospective cohort LUNG-SAFE (Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure) study, 53% of ARDS patients were ventilated with a tidal volume of less than 7 mL/kg, and 82.6% of the patients received a PEEP of less than 12 cmH2O, whilst prone positioning was used in 16.3% of patients with severe ARDS. Mechanical ventilation guidelines (43) suggest higher rather than lower levels of PEEP, taking into account that higher PEEP strategies are not associated with significant differences in barotrauma, new organ failure, ventilator-free days and mortality as compared with a lower PEEP strategy. ARDS still appears to be undertreated and associated with high mortality rate (in the LUNG-SAFE study, mortality as high as 46.1% was observed for those with severe ARDS). PEEP titration based on PaO2/FiO2 ratio may not be a correct approximation; a more complete and individually personalized understanding or ARDS lung mechanics and its interaction with the ventilation is needed (44). As Gattinoni et al. (45) discuss, in order to set tidal volume and PEEP we must keep in mind hemodynamics and lung mechanics, setting ventilator parameters knowing lung recruitment capacity (avoiding atelectrauma) and keeping the balance with an adequate tidal volume (avoiding overstretching phenomena), optimizing lung size and homogeneity.
Spontaneous breathing trials (SBT) (46-50)
Spontaneous breathing during mechanical ventilation improves lung compliance; patients preserve their end expiratory lung volumes more easily, and avoid redistribution of ventilation to the ventral parts of the lung. Atelectrauma, induced by cyclic collapse and tidal recruitment, appears to be reduced. EIT can also play an important role in demonstrating the advantages of partial ventilatory support. Moreover, EIT is capable of visualizing and quantifying spatial ventilation distribution in real time (49), taking advantage of the GI index to predict in advance whether planned SBT would lead to clinical impairment.
Prone positioning (and other positions)
Current scientific evidence derived from clinical trials and meta-analysis suggest that ventilation in the prone position should be part of the ventilatory strategy in patients with severe ARDS. EIT allows a close monitoring on changes in ventilation caused by these position changes, and contributes to assess the functional effect on the lung system in response to position changes, particularly when there is a highly unbalanced distribution of ventilation (51-53).
Clinical applications in other situations
Occasionally, abrupt decrease in regional lung ventilation may endanger the patient’s life, requiring a rapid therapeutic action to avoid worse outcomes. EIT may guide towards the diagnosis of pneumothorax, atelectasis, malpositioning of the endotracheal tube or pleural effusion (54). Though EIT imaging is not pathognomonic, it provides additional information that, along with clinical findings, contribute to achieve a prompt diagnosis. Costa et al. (55) created algorithms for the detection of this pneumothorax using the EIT, demonstrating a sensitivity of 100% for its diagnosis, even in cases of small pneumothorax (under 20 mL).
EIT may be used as a supplementary tool along with conventional pulmonary function tests, as it enables the assessment of both spatial and temporal heterogeneity of reginal function over time or after an intervention (56,57). Furthermore, EIT has also been used to assess patients with cystic fibrosis, identifying regional airway obstruction, and monitoring regional ventilation during breathing aids or physiotherapy (58-60).
Transient lung depressurization during suctioning of secretions or bronchoscopic maneuvers may be evident through EIT monitoring (61,62). Wolf et al. (63) showed that lung volumes decreased after three suctioning maneuvers during clearance of secretions, whilst Lindgren et al. (64) demonstrated that bronchoscopy initially leads to localized auto-PEEP due to the reduced transverse area available for airflow, and that suctioning leads to a decrease in lung aeration and compliance even when a closed suctioning system was used. EIT may even be able to guide lung sampling with non-bronchoscopic bronchoalveolar lavage (especially when bronchoalveolar lavage through bronchoscopy is contraindicated) (65).
Assessment of pulmonary edema often requires invasive measurements, even though they are not always reliable. New technologies can help us in this conundrum, such as lung ultrasound or EIT. In an experimental animal model (66), a novel noninvasive technique using changes in thoracic EIT with lateral body rotation was able to estimate extravascular lung water. This lung water/EIT ratio may be able to guide fluid management, while minimizing ventilator-induced lung injury.
Even though EIT has been extensively developed in lung monitoring, we must not forget to mention new lines of research, such as the assessment of cardiac function and lung perfusion (67-70). EIT-based perfusion monitoring may allow image of local perfusion and mapping of regional ventilation-perfusion ratios. Some of the approaches described to separate cardiac from respiratory impedance changes are by holding the breath, by use of modeling techniques that may separate both signals while breathing (heartbeat-related EIT signal variations) or by intravenous injection of hypertonic saline (which serves as contract agent due to its much higher conductivity). Nevertheless, clinical validation in this field is still pending.
Limitations and problems to solve
The advantages of pulmonary EIT have been reviewed. However, the constraints and limitations of this technique need to be addressed. Although it is a fairly simple technique to apply, it may pose a risk when placed in patients with spinal injury, thus it is considered a relative contraindication. The belt should not be placed over areas of damaged or inflamed skin. Poor quality EIT images could be obtained from morbid obese patients with body mass index above 50. Measurements of EIT are sensitive to body movements, hence their use in patients with uncontrolled body movements may not be reliable.
Regarding the interference of the EIT and pacemakers or defibrillators, the application of an alternating current could interfere in its functioning. Also, cardiac defibrillations should not be performed at the same time as EIT, since the energy needed for defibrillation can propagate through the electrode-belt and decrease its effectiveness.
EIT measurement encompasses cross-sectional slice of 5-10 cm of thorax, and we assume that other lung regions behave similarly. The clinical use and plausibility of EIT measurements depends then on proper belt position, proper impedance visualization, correct analysis and data interpretation (71).
The images provided translate lung tissue impedance changes, not absolute values. We will always need for a control measure (a reference) taken before or at the beginning of the morbid process to be able to determine or estimate the observed changes. Additionally, EIT captures only ventral-to-dorsal regional ventilation distribution; Bikker et al. (72) report different ventilation distribution between cranial and caudal lung levels during decremental PEEP trials, concluding that no single optimal PEEP level exists for the whole lung.
Conclusions
Despite the possible limitations of the existing EIT systems, this device have meant a remarkable technological advance in the field of lung monitoring and mechanical ventilation adjustments. The final appraisal of the indications is especially promising in the light of the first results, although its implantation on a daily basis will be the result of the scientific community’s acquired experience over the years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Theerawit P, Suthersan Y, Ball L, et al. Respiratory monitoring in adult intensive care unit. Expert Rev Respir Med 2017;11:453-68. [Crossref] [PubMed]
- Bellani G, Rouby JJ, Constantin JM, et al. Looking closer at acute respiratory distress syndrome: the role of advanced imaging techniques. Curr Opin Crit Care 2017;23:30-7. [Crossref] [PubMed]
- Chiumello D, Froio S, Bouhemad B, et al. Clinical review: Lung imaging in acute respiratory distress syndrome patients - an update. Crit Care 2013;17:243. [Crossref] [PubMed]
- Frerichs I. Electrical impedance tomography (EIT) in applications related to lung and ventilation: a review of experimental and clinical activities. Physiol. Meas 2000;21:R1-R21. [Crossref] [PubMed]
- Frerichs I, Amato MBP, Van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017;72:83-93. [Crossref] [PubMed]
- Bodenstein M, David M, Markstaller K. Principles of electrical impedance tomography and its clinical application. Crit Care Med 2009;37:713-24. [Crossref] [PubMed]
- Costa ELV, Lima RG, Amato MBP. Electrical impedance tomography. Curr Opin Crit Care 2009;15:18-24. [Crossref] [PubMed]
- Walsh BK, Smallwood CD. Electrical Impedance Tomography during Mechanical Ventilation. Respir Care 2016;61:1417-24. [Crossref] [PubMed]
- Gong B, Krueger-Ziolek S, Moeller K, et al. Electrical impedance tomography: functional imaging on its way to clinical practice? Expert Rev Respir Med 2015;9:721-37. [Crossref] [PubMed]
- Leonhardt S, Lachman B. Electrical impedance tomography: the holy grail of ventilation and perfusion monitoring? Intensive Care Med 2012;38:1917-29. [Crossref] [PubMed]
- Muders T, Luepschen H, Putensen C. Impedance tomography as a new monitoring technique. Curr Opin Crit Care 2010;16:269-75. [Crossref] [PubMed]
- Canet J, Gallart L. The Dark Side of the Lung. Unveiling Regional Lung Ventilation with Electrical Impedance Tomography. Anesthesiology 2012;116:1186-88. [Crossref] [PubMed]
- Faes TJ, van der Meij HA, de Munck JC, et al. The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas 1999;20:R1-10. [Crossref] [PubMed]
- Visser KR. Electric properties of flowing blood and impedance cardiography. Ann Biomed Eng 1989;17:463-73. [Crossref] [PubMed]
- Tomicic V, Fuentealba A, Martinez E, et al. Fundamentos de la ventilación mecanica en el síndorme de distress respiratorio agudo. Med Intensiva 2010;34:418-27. [Crossref] [PubMed]
- Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-5. [Crossref] [PubMed]
- Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome—A randomized controlled trial. JAMA 1999;282:54-61. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Bombino M, et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in Acute Respiratory Failure. Anesthesiology 1988;69:824-32. [Crossref] [PubMed]
- Wolf GK, Gómez-Laberge C, Rettig JS, et al. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med 2013;41:1296-304. [Crossref] [PubMed]
- Gentile MA, Cheifetz IM. Optimal positive end-expiratory pressure: The search for the Holy Grail continues. Crit Care Med 2004;32:2553-4. [Crossref] [PubMed]
- Crotti S, Mascheroni D, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 2001;164:131-40. [Crossref] [PubMed]
- Riera J, Riu PJ, Casan P, et al. Tomografía de impedancia eléctrica en la lesión pulmonar aguda. Med Intensiva 2011;35:509-17. [Crossref] [PubMed]
- Frerichs I, Dargaville P, Dudykevych T, et al. Electrical impedance tomography: a method for monitoring regional lung aeration and tidal volume distribution? Intensive Care Med 2003;29:2312-6. [Crossref] [PubMed]
- Kunst PW, Vazquez de Anda G, Bohm SH, et al. Monitoring of recruitment and derecruitment by electrical impedance tomography in a model of acute lung injury. Crit Care Med 2000;28:3891-5. [Crossref] [PubMed]
- van Genderingen HR, van Vught AJ, Jamsen JR. Estimation of regional volume changes by electrical impedance tomography during a pressure-volume maneuver. Intensive Care Med 2003;29:233-40. [Crossref] [PubMed]
- Hinz J, Moerer O, Neumann P, et al. Effect of positive end-expiratory -pressure on regional ventilation in patients with acute lung injury evaluated by electrical impedance tomography. Eur J Anaesthesiol 2005;22:817-25. [Crossref] [PubMed]
- Hinz J, Moerer O, Neumann P, et al. Regional pulmonary pressure-volume curves in mechanically ventilated patients with acute respiratory failure measured by electrical impedance tomography. Acta Anaesthesiol Scand 2006;50:331-9. [Crossref] [PubMed]
- Odenstedt H, Lindgren S, Olegård C, et al. Slow moderate pressure recruitment manoeuvre minimizes negative circulatory and lung mechanic side effects: evaluation of recruitment manoeuvres using electric impedance tomography. Intensive Care Med 2005;31:1706-14. [Crossref] [PubMed]
- Lowhagen K, Lindgren S, Odensteddt H, et al. A new non-radiological method to assess potential lung recruitability: a pilot study in ALI patients. Acta Anaesthesiol Scand 2011;55:165-74. [Crossref] [PubMed]
- Putensen C. Electrical impedance tomography guided ventilation therapy. Curr Opin Crit Care 2007;13:344-50. [Crossref] [PubMed]
- Luepschen H, Meier T, Grossherr M, et al. Protective ventilation using electrical impedance tomography. Physiol Meas 2007;28:S247-60. [Crossref] [PubMed]
- Wolf GK, Gómez-Laberge C, Rettig JS, et al. Mechanical ventilation guided by electrical impendance tomography in experimental acute lung injury. Crit Care Med 2013;41:1296-304. [Crossref] [PubMed]
- Costa EL, Batista BJ, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009;35:1132-7. [Crossref] [PubMed]
- Bikker IG, Leonhardt S, Bakker J, et al. Lung volume calculated from electrical impedance tomography in ICU patients at different PEEP levels. Intensive Care Med 2009;35:1362-7. [Crossref] [PubMed]
- Zhao Z, Moller K, Steinmann D, et al. Evaluation of an electrical impedance tomography-based Global Inhomogeneity Index for pulmonary ventilation distribution. Intensive Care Med 2009;35:1900-6. [Crossref] [PubMed]
- Zhao Z, Steinmann D, Frerichs I, et al. PEEP titration guided by ventilation homogeneity: a feasibility study using electrical impedance tomography. Crit Care 2010;14:R8. [Crossref] [PubMed]
- Liu S, Tan L, Möller K, et al. Identification of regional overdistension, recruitment and cyclic alveolar collapse with electrical impedance tomography in an experimental ARDS model. Crit Care 2016;20:119. [Crossref] [PubMed]
- Eronia N, Mauri T, Maffezzini E, et al. Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: a feasibility study. Ann Intensive Care 2017;7:76. [Crossref] [PubMed]
- Franchineau G, Bréchot N, Lebreton G, et al. Bedside contribution of electrical impedance tomography to set positive end-expiratory pressure for ECMO-Treated Severe ARDS Patients. Am J Respir Crit Care Med 2017;196:447-57. [Crossref] [PubMed]
- Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011;107:998-1004. [Crossref] [PubMed]
- Riera J, Pérez P, Cortés J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 2013;58:589-96. [Crossref] [PubMed]
- Bellani G, Laffeyu JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of critical Care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017;195:1253-63. [Crossref] [PubMed]
- Gordo F, Conejo I. What PEEP level should I use in my patient? Med Intensiva 2017;41:267-9. [Crossref] [PubMed]
- Gattinoni L, Marini JJ, Collino F, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care 2017;21:183. [Crossref] [PubMed]
- Blankman P, Hasan D, van Mourik MS, et al. Ventilation distribution measured with EIT at varying levels of pressure support and neurally adjusted ventilatory assist in patients with ALI. Intensive Care Med 2013;39:1057-62. [Crossref] [PubMed]
- Radke OC, Shneider T, Heller AR, et al. Spontaneous breathing during general anesthesia prevents the ventral redistribution of ventilation as detected by electrical impedance tomography a randomized trial. Anesthesiology 2012;116:1227-34. [Crossref] [PubMed]
- Spaeth J, Daume K, Goebel U, et al. Increasing positive end-expiratory pressure (re-)improves intraoperative respiratory mechanics and lung ventilation after prone positioning. Br J Anaesth 2016;116:838-46. [Crossref] [PubMed]
- Bickenbach J, Czaplik M, Polier M, et al. Electrical impedance tomography for predicting failure of spontaneous breathing trials in patients with prolonged weaning. Crit Care 2017;21:177. [Crossref] [PubMed]
- Hsu YL, Tien AJ, Chang MY, et al. Regional Ventilation Redistribution Measured by Electrical Impedance Tomography during Spontaneous Breathing Trial with Automatic Tube Compensation. Physiol Meas 2017;38:1193-203. [Crossref] [PubMed]
- Mora-Arteaga J, Bernal Ramírez OJ, Rodríguez SJ, et al. Efecto de la ventilación mecánica en posición prona en SDRA. Una revisión sistemática y metaanálisis. Med Intensiva 2015;39:352-65. [Crossref]
- Bein T, Ploner F, Ritzka M, et al. No change in the regional distribution of tidal volume during lateral posture in mechanically ventilated patients assessed by electrical impedance tomography. Clin Physiol Funct Imaging 2010;30:234-40. [Crossref] [PubMed]
- Gordo F, Hermosa C. Physiology and evidence join in favor or prone decubitus. Med Intensiva 2015;39:327-8. [Crossref] [PubMed]
- Arad M, Zlochiver S, Davidson T, et al. The detection of pleural effusion using a parametric EIT technique. Physiol Meas 2009;30:421-8. [Crossref] [PubMed]
- Costa ELV, Chaves CN, Gomes S, et al. Real-time detection of pneumothorax using electrical impedance tomography. Crit Care Med 2008;36:1230-8. [Crossref] [PubMed]
- Vogt B, Pulletz S, Elke G, et al. Spatial and temporal heterogeneity of regional lung ventilation determined by electrical impedance tomography during pulmonary function testing. J Appl Physiol (1985) 2012;113:1154-61. [PubMed]
- Frerichs I, Achtzehn U, Pechmann A, et al. High-frequency oscillatory ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease. J Crit Care 2012;27:172-81. [Crossref] [PubMed]
- Zhao Z, Fischer R, Frerichs I, et al. Regional ventilation in cystic fibrosis measured by electrical impedance tomography. J Cyst Fibros 2012;11:412-8. [Crossref] [PubMed]
- Zhao Z, Muller-Lisse U, Frerichs I, et al. Regional airway obstruction in cystic fibrosis determined by electrical impedance tomography in comparison with high resolution CT. Physiol Meas 2013;34:N107-14.
- Wettstein M, Radlinger L, Riedel T. Effect of different breathing aids on ventilation distribution in adults with cystic fibrosis. PLoS One 2014;9:e106591. [Crossref] [PubMed]
- Lindgren S. Regional lung derecruitment after endotracheal suction during volume or pressure-controlled ventilation: a study using electric impedance tomography. Intensive Care Med 2007;33:172-80. [Crossref] [PubMed]
- Corley A, Spooner AJ, Barnett AG, et al. End-expiratory lung volume recovers more slowly after closed endotracheal suctioning than after open suctioning: a randomized crossover study. J Crit Care 2012;27:742.e1-7. [Crossref] [PubMed]
- Wolf GK, Grychtol B, Frerichs I, et al. Regional lung volume changes in children with acute respiratory distress syndrome during a derecruitment maneuver. Crit Care Med 2007;35:1972-8. [Crossref] [PubMed]
- Lindgren S, Odenstedt H, Erlandsson K, et al. Bronchoscopic suctioning may cause lung collapse: a lung model and clinical evaluation. Acta Anaesthesiol Scand 2008;52:209-18. [Crossref] [PubMed]
- Grieco DL, Mura B, Bisanti A, et al. Electrical impedance tomography to monitor lung sampling during broncho-alveolar lavage. Intensive Care Med 2016;42:1088-9. [Crossref] [PubMed]
- Trepte CJC, Phillips CR, Solà J, et al. Electrical impedance tomography for quantification of pulmonary edema in acute lung injury. Crit Care 2016;20:18. [Crossref] [PubMed]
- Deibele JM, Luepschen H, Leonhardt S. Dynamic separation of pulmonary and cardiac changes in electrical impedance tomography. Physiol Meas 2008;29:S1-S14. [Crossref] [PubMed]
- Grant CA, Pham T, Hough J, et al. Measurement of ventilation and cardiac related impedance changes with electrical impedance tomography. Crit Care 2011;15:R37. [Crossref] [PubMed]
- Borges JB, Suarez-Sipmann F, Bohm SH, et al. Regional lung perfusion estimated by electrical impendance tomography in a piglet model of lung collapse. J Appl Physiol (1985) 2012;122:225-36.
- Frerichs I, Becher T, Weiler N. Electrical impedance tomography imaging of the cardiopulmonary system. Curr Opin Crit Care 2014;20:323-32. [Crossref] [PubMed]
- Karsten J, Stueber J, Voigt N, et al. Influence of different electrode belt positions on electrical impedance tomography imaging of regional ventilation: a prospective observational study. Crit Care 2016;20:3. [Crossref] [PubMed]
- Bikker IG, Preis C, Egal M, et al. Electrical impedance tomography measured at two thoracic levels can visualize the ventilation distribution changes at the bedside during a decremental positive end-expiratory lung pressure trial. Crit Care 2011;15:R193. [Crossref] [PubMed]


