Poly(GP) proteins: a potential pharmacodynamic marker in ALS and FTD
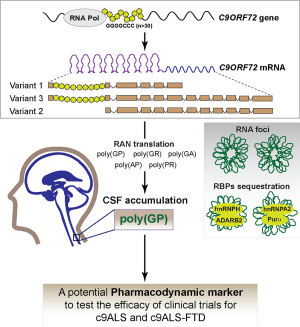
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord. The genetic factor chromosome 9 open reading frame 72, C9ORF72, has been closely associated not only with the development of ALS, but also with frontotemporal dementia (FTD) characterized by a progressive cognitive decline, altered personality and impairment in language (1,2). The C9ORF72 gene can generate three different transcript variants (C9orf72 V1, V2 and V3) by alternative splicing of its pre-mRNA and harbors different repeats of the hexanucleotide GGGGCC [(G4C2)n]. This G4C2 repeat expansion, located within the intron that separates exons 1a and 1b (3,4), is present in a number of 20 or less G4C2 repetitions (n≤20) in healthy people. However, C9ORF72-associated ALS (c9ALS) or FTD (c9FTD) individuals have several hundred to several thousand repeats (5). Abnormal accumulation of sense and antisense transcripts derived from G4C2 repeat expansions cause RNA foci formation with the concomitant sequestration of RNA-binding proteins (RBPs), such as hnRNPH, ADARB2, hnRNPA2 and Pura (6). In addition, RNA foci can also undergo repeat-associated non-ATG (RAN) translation both in the sense and antisense transcripts, generating Poly(GP), poly(GA), and poly(GR) proteins, also known as c9RAN proteins (7). Studies have shown that the aberrant accumulation of these c9RAN proteins results to be toxic and contributes to the progressive neuronal degeneration (7,8) characteristic of ALS and FTD.
Despite multiple clinical trials have been performed, current pharmacological interventions only have shown poor effectiveness against c9ALS, c9FTD or c9ALS-FTD. To face this situation, not only is critical the understanding of the mechanisms regulating ALS or FTD, but also the identification of accurate pharmacodynamic markers. In line with this, the elegant work of Gendron and colleagues recently published in Science Translational Medicine (doi: 10.1126/scitranslmed.aai7866) (9) explores the possibility of using poly(GP) proteins as an endogenous pharmacodynamic marker, which they might be used as powerful tool to validate the efficacy of novel or established clinical trials for c9ALS.
Gendron and colleagues were able to detect a significant increase in the level of poly(GP) polypeptide in the cerebrospinal fluid (CSF) from both asymptomatic and symptomatic C9ORF72 repeat expansion carriers. In addition, the concentration of poly(GP) proteins evaluated in CSF from asymptomatic C9ORF72 mutation carriers and patients with c9ALS or c9ALS-FTD showed to be largely constant. In this regard, Gendron and colleagues showed that poly(GP) proteins are consistently found, in a stable and individual-dependent concentration, in the CSF of patients, for a period of time of up to 22.6 months since the first CSF collection, in a total of 33 subjects (9 asymptomatic C9ORF72 mutation carriers and 24 c9ALS or c9ALS-FTD individuals). Importantly, detection of Poly(GP) proteins in the CSF cannot be considered as a prognostic marker since the authors did not observe a significant association between the levels of the poly(GP) in the CSF and the age at the onset of the disease, onset site (bulbar, limb, or other) or type of disease. In addition, no correlations were found when the levels of poly(GP) where analyzed in the CSF and associated with survival after disease onset in patients with c9ALS or c9ALS-FTD. Indeed, there was no difference in poly(GP) protein levels from c9ALS or c9ALS-FTD individuals with or without behavioral impairment. Thus, given the promising potential use of poly(GP) as a pharmacodynamic marker in C9ORF72-associated ALS and ALS-FTD, the authors evaluated whether the presence of poly(GP) proteins could be used to evaluate the effectiveness of therapeutic strategies addressed against G4C2 repeat-containing transcripts. Using an elegant approach, Gendron et al. showed that antisense oligonucleotides (ASO), targeted against G4C2 repeat RNA (c9ASO), decrease both intracellular and secreted-to-media poly(GP) proteins in immortalizing peripheral blood mononuclear cells (PBMCs) from C9ORF72 mutation carriers. The same results were obtained using c9ALS induced pluripotent stem cells (iPSCs)-derived neurons. Importantly, c9ASOs decreased the level of G4C2 repeat-containing transcripts, identified as C9ORF72 variants 1 and 3, while they did not affect the amount of the variant 2, which does not carry the G4C2 repeat. These data showed an interesting correlation between high intra and extracellular levels of poly(GP) proteins and the accumulation of intracellular G4C2 repeat-containing transcripts. In this regard, the work of Gendron and colleagues give us convincing evidence indicating that extracellular and CSF poly(GP) proteins represent a useful candidate as a pharmacodynamic marker for therapies focused on reducing the abnormal accumulation of G4C2 RNA. Furthermore, the authors evaluated the efficacy of extracellular poly(GP) as a pharmacodynamic marker in vivo. Indeed, they evaluated the effect of c9ASO on poly(GP) accumulation in a murine model previously validated by Chew and colleagues, where they overexpressed an expanded (G4C2)66 repeat which causes neuronal damage and behavioral impairment mimicking TAR DNA-binding protein-43 (TDP-43)-associated-neurodegeneration (10). c9ASO or vehicle alone was injected in the right ventricle of the central nervous system in 16–18 week old control (G4C2)2 mice and in mice carrying the (G4C2)66 repeat. In this experiment, the authors detected high levels of poly(GP) in the CSF from (G4C2)66 animals, which were significantly blunted when the animals were injected with c9ASO. Accordingly with the data above, Gendron et al. also observed that the presence of RNA foci, inclusions for poly(GR), poly(GA) or poly(GP) accumulated in motor cortex tissues from animals (G4C2)66 were significantly decreased when the animals were treated with c9ASO. To note, in the aforementioned experiment the authors did not evaluate if c9ASO treatment is also able to improve the behavior of (G4C2)66 mice, an important question that should be answered by future studies. In summary, the work of Gendron and colleagues demonstrates that poly(GP) protein levels correlate with G4C2 repeat expansion RNA in vivo. This work indicates poly(GP) proteins represent a promising pharmacodynamic marker to test the effectiveness of therapies for c9ALS, c9FTD or C9ORF72 repeat expansions-related diseases in forthcoming clinical trials. However, we need to remember that also other proteins, such as TDP-43 and superoxide dismutase-1 (SOD-1), are implicated in these diseases and that only the 10% of all ALS cases are the consequence of a genetic mutation (11), thus suggesting other pharmacodynamic markers need to be identified in the years to come.
In conclusion, this study increases our understanding about the pathogenesis of the most common genetic cause of ALS, and, through in vitro and in vivo assays, identifies poly(GP) as a suitable option for the clinical testing of therapies for ALS and FTD and other diseases associated with C9ORF72 repeat expansions [the Figure 1 summarizes the main aspects exposed by Gendron and colleagues (9)]. Reliable and reproducible pharmacodynamic markers are needed for the clinical testing of innovative and conventional therapeutics for subjects with C9ORF72 repeat expansions-associated diseases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- Cruts M, Gijselinck I, Van Langenhove T, et al. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 2013;36:450-9. [Crossref] [PubMed]
- Budini M, Buratti E, Morselli E, et al. Autophagy and Its Impact on Neurodegenerative Diseases: New Roles for TDP-43 and C9orf72. Front Mol Neurosci 2017;10:170. [Crossref] [PubMed]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245-56. [Crossref] [PubMed]
- Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72:257-68. [Crossref] [PubMed]
- van Blitterswijk M, DeJesus-Hernandez M, Niemantsverdriet E, et al. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol 2013;12:978-88. [Crossref] [PubMed]
- Lee YB, Chen HJ, Peres JN, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep 2013;5:1178-86. [Crossref] [PubMed]
- Lee KH, Zhang P, Kim HJ, et al. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell 2016;167:774-788.e17. [Crossref] [PubMed]
- Gendron TF, Belzil VV, Zhang YJ, et al. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol 2014;127:359-76. [Crossref] [PubMed]
- Gendron TF, Chew J, Stankowski JN, et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci Transl Med 2017;9:eaai7866. [Crossref] [PubMed]
- Chew J, Gendron TF, Prudencio M, et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science 2015;348:1151-4. [Crossref] [PubMed]
- Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature 2016;539:197-206. [Crossref] [PubMed]


