D-dimer level is related to the prognosis of patients with small cell lung cancer
Introduction
One of the typical characteristics of cancer is an imbalance of the coagulation system, although the exact molecular mechanism remains unknown (1). Malignancy can interfere with the status of the haemostatic system, and conversely, the haemostatic system can affect the biological behaviour of the malignancy. Previous studies have demonstrated that haemostatic abnormalities exist in cancer patients (2). For instance, the coagulation system in some cancer patients is over-activated. The over-activated coagulation system combined with the activated fibrinolysis system promotes clot degradation and increases fibrin degradation. These changes impart an increased tendency for both thrombosis and haemorrhage in these patients. Consequently, thromboembolism frequently occurs in cancer patients. A process of fibrin formation and fibrinolysis is often paralleled with the development of malignancy (3), leading to enhanced tumour angiogenesis (4), poor prognosis (5), and even death in these patients (6).
D-dimer is the lysis product of cross-linked fibrin that is formed by coagulation system activation, which signals hyperfibrinolysis in response to clot activation and fibrin formation, although its role in cancer development is not well understood (7). In the past decade, the prognostic value of the D-dimer level in patients with colorectal cancer (8), ovarian cancer (9), and gastric cancer (10,11) has attracted much attention. In most of these studies, D-dimer levels were found to be elevated and correlated with the stage and prognosis of the disease. Therefore, the D-dimer level is considered a promising prognostic predictor.
D-dimer levels were also found to be elevated in patients with lung cancer (12-14) and correlated to a poor prognosis (15). However, those studies included only a limited number of small cell lung cancer (SCLC) patients. The prognostic value of D-dimer in SCLC remains poorly understood. It is well known that biological behaviours, including the ability to metastasise, are quite different between non-SCLC and SCLC. Those behaviours can affect the prognosis of patients remarkably. Although a few tumour markers have proven useful in estimating the prognosis of SCLC patients, their prognostic value is limited; therefore, more prognostic predictors are urgently needed to better predict the prognosis of SCLC (16). Therefore, we performed this study to determine the prognostic significance of D-dimer levels in SCLC patients.
Methods
Materials
The medical records of newly diagnosed SCLC patients who were admitted to Anhui Provincial Hospital between January 2010 and August 2013 were retrospectively reviewed. All enrolled patients were pathologically confirmed as SCLC. Patients who had any of the following conditions were excluded: (I) complicated with malignant disease other than SCLC; (II) end-stage renal or liver disease; (III) life expectancy shorter than 1 month; (IV) inherited thrombosis or homeostasis disorders; (V) received anti-thrombosis treatment in the past 3 months for any of the following diseases: cerebral infarction, cardiovascular infarction, (VI) pulmonary embolism, and vein embolism; (VII) infection; or (VIII) received operation within past two weeks. Patients without sufficient data were also excluded. The study, involved no interventions on management of the patients, was approved by the Ethic Committee of Anhui Provincial Hospital and an informed consent was received from each patient or his/her close relatives.
Data extraction
We extracted the following data from the patient’s record: age, gender, smoking status, date of the diagnosis, Eastern Cooperative Oncology Group performance status (ECOG-PS), stage (classified as limited or extensive), and plasma D-dimer level. To explore the correlation between D-dimer levels and other known prognostic predictors, we also extracted the data of two often-used tumour markers, serum neuron-specific enolase (NSE) and carcinoembryonic antigen (CEA) levels. D-dimer was determined using Sysmex CA-1500 (Sysmex Corporation, Kobe, Japan), CEA and NSE were determined using Cobas e601 (Roche Diagnostics, Numbrecht, Germany). All of these values were available in examination results performed before treatment. These data were regarded as the baseline characteristics of the patients.
Follow-up
The endpoint of the present study was SCLC-related mortality. All eligible patients were followed up in the end of December 2013. For some patients who completed their treatment in our hospital, the endpoint was extracted from medical records. Otherwise, many attempts were made to contact the patients or their close relatives by telephone to obtain the information of their current survival status. The overall survival was defined as the period between the time of diagnosis to the date of SCLC-related death or the last contact. The survival time and the status of alive or not at the closure of this study were recorded for all of the patients.
Data analysis
The distribution of the data was tested by Kolmogorov-Smirnov test. Median and quartiles were used to describe continuous data and Student’s t-test or the Mann-Whitney test was used for their comparison. The forward conditional Cox hazards regression model was used to determine the correlation between the D-dimer levels and the risk for mortality. To evaluate the correlation between the D-dimer levels and tumour-related mortality, we divided the patients into two groups, normal (<0.55 mg/L) and abnormal (their treat groups, and applied Kaplan-Meier survival curves and the log-rank test for analysis. The Spearman approach was employed to assess the relationship between two continuous data points. All statistical analyses were performed with SPSS 13.0 software (a statistical package), and a P value less than 0.05 was regarded as statistically significant. All reported P values are two-tailed.
Results
Clinical characteristic of the patients
A total of one hundred and seven patients with SCLC were ultimately enrolled in the present study. The characteristics of the patients are shown in Table 1. The median D-dimer level was 0.59 mg/L (quartiles: 0.33–1.10), which was higher than that of the normal estimate (0.55 mg/L). Most of the patients (87.85%) were cigarette smokers. Sixty-five (60.7%) were diagnosed at extensive stages. The median follow-up duration was 9 months (quartiles: 6–16 months), and 91 (85.0%) subjects died during the follow-up period.

Full table
The correlation between D-dimer level and clinical characteristics
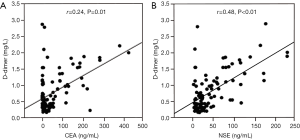
Abnormally high D-dimer levels were observed in patients at extensive stage SCLC compared with those at limited stage (Figure 1). The difference between the two groups was statistically significant (P<0.02). In analyses of the correlation between D-dimer level and age, gender, haemoglobin, albumin and ECOG-PS, no positive results were found. Plasma D-dimer levels were positively correlated with CEA and NSE levels, with a correlation coefficient of 0.24 (P=0.01) and 0.48 (P<0.01), respectively (Figure 2A,B).

D-dimer level and patient survival
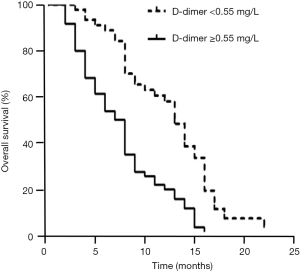
At the end of follow up, the mortality rates of the two groups were significantly different, as 37 patients (80.43%) with normal levels and 54 patients (88.52%) with elevated D-dimer levels died during follow-up. Patients with elevated D-dimer levels (≥0.55 mg/L, the upper limit of reference interval) had a shorter median survival time than those with normal levels (8 vs. 16 months, respectively). Kaplan-Meier survival analysis further demonstrated that the survival time for SCLC patients with elevated D-dimer levels was significantly reduced compared with those with normal levels (P<0.01 by log-rank test, Figure 3). Furthermore, to evaluate the prognostic value of D-dimer level for SCLC prognosis estimation, a Cox hazard regression approach was employed. As shown in Table 2, in a univariate model, an elevated D-dimer level (≥0.55 mg/L) was significantly associated with SCLC-related mortality, with a hazard ratio (HR) of 2.59 (95% CI: 1.65–4.05). The HR value remained significant when adjusted for NSE, CEA and tumour stage, with an HR of 2.14 (95% CI: 1.34–3.41).

Full table
Discussion
Once the diagnosis of SCLC is established, the prognostic estimation for the patient becomes especially crucial because it can greatly affect management. Compared with imaging and cytological approaches, laboratory tests are inexpensive and convenient. They can be repeated before, during, and after chemotherapy. Certain laboratory biomarkers have been evaluated for predicting the prognosis of cancer (17-19). Indeed, although several pre-treatment serum prognostic factors for SCLC have been found, prognostic estimation continues to be a challenge for physicians in daily practice (17). It remains necessary to explore novel markers to facilitate the prognosis estimation for those patients.
Haemostatic abnormality is common in cancer patients (20-22). Subjects with disturbed haemostatic status usually have a poor prognosis (2,20). D-dimer, one of the key components in the process of embolism and fibrinolysis, has been found to be associated with the prognosis in patients with lung cancer (23). Several studies have reported that plasma D-dimer levels were elevated and associated with the stage and mortality in lung cancer (14,23,24). Most of the subjects enrolled in those studies were patients with non-SCLC. Whether D-dimer levels are associated with the prognosis of SCLC is not clear. In our study, which enrolled only patients with SCLC, elevated D-dimer levels were confirmed. The results are consistent with those of other reports (16,25). However, compared with these two recently published works, the strength of our work is that some confounding factors, such as NSE, albumin and haemoglobin are adjusted. While these factors were not considered by previous studies. In addition, our results demonstrated that patients at extensive stage had higher plasma D-dimer levels than those at limited stage. The mortality was significantly higher in patients with elevated D-dimer levels than in those with normal levels as identified by Kaplan-Meier survival curves and Cox proportional hazards regression analysis. This implies that D-dimer levels are correlated with patient survival and could be a potentially prognostic predictor for SCLC.
Certain tumour markers have been proven to be of prognostic value in lung cancer. For instance, NSE, a sensitive tumour marker for SCLC, is used in predicting the patient outcomes (26). The pre-treatment NSE levels are reported to be elevated in SCLC patients and are significantly higher when the patients are at extensive stage. Patients with high levels of NSE have a shorter overall survival compared with those with normal levels, as summarised by a review (27). Therefore, NSE has been routinely used as an independent prognostic predictor for SCLC (2,20). CEA, another frequently used tumour marker, is also elevated in lung cancer patients. Research has revealed that SCLC patients with high CEA levels had a significantly shorter survival time than those with normal CEA levels (28), which indicates that CEA is also an independent prognostic factor for SCLC (29). In our study, both NSE and CEA levels were found to be elevated and positively correlated with D-dimer levels. Because NSE and CEA are well-recognised prognostic factors, these results further indicated the value of D-dimer as a useful prognostic predictor in SCLC, as suggested by the consistency between D-dimer, NSE and CEA levels.
Our study has some limitations. This is a retrospective observational study with small sample size. Therefore, the findings of this study need to be validated by prospective and multicentre studies. Second, the treatment approaches for subjects are various; therefore, the confounding effects of treatment approaches may bias the results. Further studies are needed to rigorously evaluate the prognostic value of D-dimer in subjects received a specific treatment approach.
In conclusion, our study indicates that SCLC patients with increased D-dimer levels have a shorter survival time. Evaluation of the pre-treatment D-dimer level is useful in estimating the prognosis of patients with SCLC.
Acknowledgements
The authors are grateful to staff members of the Medical Records Room, Anhui Provincial Hospital, for their help in the data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Anhui Provincial Hospital and written informed consent was obtained from all patients.
References
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013;11:223-33. [Crossref] [PubMed]
- Tas F, Kilic L, Serilmez M, et al. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med 2013;107:451-7. [Crossref] [PubMed]
- Falanga A, Marchetti M, Vignoli A, et al. Clotting mechanisms and cancer: implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol 2003;1:673-8. [PubMed]
- Nash GF, Walsh DC, Kakkar AK. The role of the coagulation system in tumour angiogenesis. Lancet Oncol 2001;2:608-13. [Crossref] [PubMed]
- Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012;97:1158-64. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4. [Crossref] [PubMed]
- Blombäck B, Hessel B, Hogg D, et al. A two-step fibrinogen--fibrin transition in blood coagulation. Nature 1978;275:501-5. [Crossref] [PubMed]
- Kilic M, Yoldas O, Keskek M, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis 2008;10:238-41. [Crossref] [PubMed]
- Koh SC, Khalil R, Lim FK, et al. The association between fibrinogen, von Willebrand Factor, antithrombin III, and D-dimer levels and survival outcome by 36 months from ovarian cancer. Clin Appl Thromb Hemost 2006;12:3-8. [Crossref] [PubMed]
- Kwon HC, Oh SY, Lee S, et al. Plasma levels of prothrombin fragment F1+2, D-dimer and prothrombin time correlate with clinical stage and lymph node metastasis in operable gastric cancer patients. Jpn J Clin Oncol 2008;38:2-7. [Crossref] [PubMed]
- Liu L, Zhang X, Yan B, et al. Elevated plasma d-dimer levels correlate with long term survival of gastric cancer patients. PLoS One 2014;9:e90547. [Crossref] [PubMed]
- Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007;19:494-8. [Crossref] [PubMed]
- Guadagni F, Ferroni P, Basili S, et al. Correlation between tumor necrosis factor-alpha and D-dimer levels in non-small cell lung cancer patients. Lung Cancer 2004;44:303-10. [Crossref] [PubMed]
- İnal T, Anar C, Polat G, et al. The prognostic value of D-dimer in lung cancer. Clin Respir J 2015;9:305-13. [Crossref] [PubMed]
- Zhou YX, Yang ZM, Feng J, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: a meta-analysis. Tumour Biol 2013;34:3701-4. [Crossref] [PubMed]
- Chen Y, Yu H, Wu C, et al. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother 2016;81:210-7. [Crossref] [PubMed]
- Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: opportunities for improvement. Biochim Biophys Acta 2013;1836:255-72. [PubMed]
- PPetrović M, Bukumirić Z, Zdravković V, et al. The prognostic significance of the circulating neuroendocrine markers chromogranin A, pro-gastrin-releasing peptide, and neuron-specific enolase in patients with small-cell lung cancer. Med Oncol 2014;31:823. [Crossref] [PubMed]
- Quoix E, Purohit A, Faller-Beau M, et al. Comparative prognostic value of lactate dehydrogenase and neuron-specific enolase in small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer 2000;30:127-34. [Crossref] [PubMed]
- Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J 2001;17:667-73. [Crossref] [PubMed]
- Iversen LH, Thorlacius-Ussing O. Systemic coagulation reactivation in recurrence of colorectal cancer. Thromb Haemost 2003;89:726-34. [PubMed]
- Koh SC, Tham KF, Razvi K, et al. Hemostatic and fibrinolytic status in patients with ovarian cancer and benign ovarian cysts: could D-dimer and antithrombin III levels be included as prognostic markers for survival outcome? Clin Appl Thromb Hemost 2001;7:141-8. [Crossref] [PubMed]
- Komurcuoglu B, Ulusoy S, Gayaf M, et al. Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori 2011;97:743-8. [PubMed]
- Buccheri G, Ferrigno D, Ginardi C, et al. Haemostatic abnormalities in lung cancer: prognostic implications. Eur J Cancer 1997;33:50-5. [Crossref] [PubMed]
- Zhu LR, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol 2016;18:178-88. [Crossref] [PubMed]
- Mumbarkar PP, Raste AS, Ghadge MS. Significance of tumor markers in lung cancer. Indian J Clin Biochem 2006;21:173-6. [Crossref] [PubMed]
- Zhao WX, Luo JF. Serum neuron-specific enolase levels were associated with the prognosis of small cell lung cancer: a meta-analysis. Tumour Biol 2013;34:3245-8. [Crossref] [PubMed]
- Bandoh S, Fujita J, Ueda Y, et al. Expression of carcinoembryonic antigen in peripheral- or central-located small cell lung cancer: its clinical significance. Jpn J Clin Oncol 2001;31:305-10. [Crossref] [PubMed]
- Yang X, Wang D, Yang Z, et al. CEA is an independent prognostic indicator that is associated with reduced survival and liver metastases in SCLC. Cell Biochem Biophys 2011;59:113-9. [Crossref] [PubMed]


