Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different
Developmental hemostasis is a concept that is now well accepted amongst experts in the field of hemostasis, but which is potentially under-recognized by many clinicians and scientists working in the field of medicine and diagnostics. Developmental hemostasis as regards to secondary hemostasis, meaning the ‘blood clotting’ aspects that involve the many coagulation factors, the often-called coagulation ‘cascade’, and the eventual conversion of fibrinogen to fibrin, was spearheaded by Andrew et al. (1,2), and more recently confirmed and formalized by Monagle, Ignjatovic and colleagues (3,4). For example, (I) the concentration of vitamin K dependent coagulation factors, thrombin generation and clot lysis are all reduced in neonates compared with older children; (II) for the natural anticoagulants, the level of antithrombin is reduced in neonates, but reaches that of adults after around six months, whereas, in contrast, protein S and protein C is markedly reduced in neonates and both increase throughout childhood, although levels do not reach those of adults until adolescence (Table 1). Indeed, the concept of hemostasis developing from the very young, including newborns and infants, onwards to childhood, and then adulthood, and even further as adults age, is now a well-established fact (5,6).
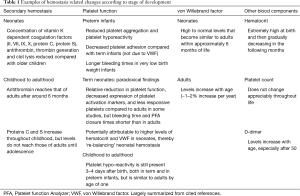
Full table
The situation with primary hemostasis, meaning aspects of platelet function (adhesion and aggregation) and involvement of von Willebrand factor (VWF) and subendothelial cell components is less well recognized but also emerging (7,8). Both reduced platelet aggregation and platelet hyporeactivity have been reported in premature infants, and preterm infants have demonstrated decreased platelet adhesion compared with term infants. Despite this platelet hyporeactivity in neonates, studies using whole blood platelet function testing employing the platelet function analyzer-100 (PFA-100) report shorter closure times in term neonates than in adult blood, and therefore suggesting a paradoxical enhanced platelet aggregation in neonates. However, this might be attributed to higher hematocrit levels and VWF concentration in neonates, thus suggesting a kind of rebalanced primary hemostasis. Interestingly, platelet function in healthy children beyond one year of age becomes similar to that of adults.
Changes or differences in hemostasis can even be recognized in adults. For example, levels of VWF in adults increase with aging, at a rate of 1–2% per year (9). A similar increase in D-dimers occurs with aging (10). Hemostasis changes are also observed in pregnancy, such that substantial changes occur that lead to an overall pro-hemostatic environment (11); in summary, most coagulation factors increase, as does VWF and D-dimer, but Protein S falls.
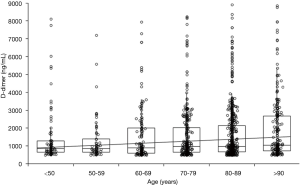
These developmental changes carry several important implications. First, reference ranges, as used by laboratories to define ‘normality’, should be appropriate to the population under investigation. Essentially, then, an adult reference range may not always be appropriate for infants, and indeed may not always be appropriate for all adults. Second, changes may alter the relative risk of bleeding or thrombosis. For example, in neonates, there is a reduced level of hemostasis that may predispose some to increased bleeding risk. In contrast, in the aging adult, hemostasis changes instead lead to a more prothrombotic milieu. A higher prothrombotic risk can also be ascribed for pregnancy. Relevant also is the diagnosis of bleeding disorders. Most simply, a bleeding disorder may be inappropriately diagnosed in the very young if an adult reference range is used. As another example, von Willebrand disease (VWD) is diagnosed as a deficiency or defect in VWF. Thus, it is quite feasible that VWD may be diagnosed at some stage in a person’s life, and by some older age, the diagnosis may ‘disappear’ because VWF levels normalize. Finally, such changes also have implications for identification of acute thrombosis. Thus, use of standard adult ranges for D-dimer will not provide optimal negative exclusion of a venous thromboembolism in older adults or in pregnant women. The former situation is clearly demonstrated in Figure 1, which shows that D-dimer values, as measured in a population of 1,447 patients admitted to the emergency department with final diagnoses other than venous thromboembolism (12), consistently increase with age [Spearman’s correlation, 0.09; 95% confidence interval (CI), 0.04–0.14; P<0.001].
This leads us to some discussion of the current approach for establishing reference ranges of hemostasis tests and other laboratory parameters, which has many under-recognized limitations. Too often the calculation is based on the so-called “reference population”, presuming that the reference subjects utilized are all “ostensibly healthy”, thus inevitably leading to collection of inaccurate data. Indeed, how can we identify that these subjects are really healthy? This is commonly inferred after collection of medical history and physical examination, which fails to show ‘abnormalities’, but which will never be able to fully exclude the ‘silent pathologies’ that are sometimes present, thus contributing to introduce a significant bias in the calculation. Although laboratories conventionally use “statistical acrobatics” to exclude outliers (i.e., cutting the tails of the value distribution and conventionally including only the 95% CI of the entire population), no reliable evidence has ever been provided to ensure that this approach is absolutely foolproof. For example, D-dimer values are increased in patients with cancer, even silent or occult, and this is something that even a total body computed tomography (CT) may not be able to fully exclude. Even if it could, it is obviously not feasible to perform a total body CT on otherwise healthy subjects merely for defining a reference range.
The take-home message of the above dissertation is that personalized medicine is the mainstay of future laboratory medicine (13), wherein longitudinal collection of personal data will be the most reliable benchmark for the diagnosis of hemostasis and other physiological disturbances.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood 1987;70:165-72. [PubMed]
- Andrew M, Vegh P, Johnston M, et al. Maturation of the hemostatic system during childhood. Blood 1992;80:1998-2005. [PubMed]
- Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 2006;95:362-72. [PubMed]
- Ignjatovic V, Kenet G, Monagle P, et al. Developmental hemostasis: recommendations for laboratories reporting pediatric samples. J Thromb Haemost 2012;10:298-300. [Crossref] [PubMed]
- Lippi G, Franchini M, Montagnana M, et al. Coagulation testing in pediatric patients: the young are not just miniature adults. Semin Thromb Hemost 2007;33:816-20. [Crossref] [PubMed]
- Favaloro EJ, Franchini M, Lippi G., et al. Aging hemostasis: changes to laboratory markers of hemostasis as we age - a narrative review. Semin Thromb Hemost 2014;40:621-33. [Crossref] [PubMed]
- Hvas AM, Favaloro EJ. Platelet function testing in pediatric patients. Expert Rev Hematol 2017;10:281-8. [Crossref] [PubMed]
- Lippi G, Manzato F, Franchini M, et al. Establishment of reference values for the PFA-100 platelet function analyzer in pediatrics. Clin Exp Med 2001;1:69-70. [Crossref] [PubMed]
- Konkle BA. Von Willebrand factor and aging. Semin Thromb Hemost 2014;40:640-4. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Cervellin G. A review of the value of D-dimer testing for prediction of recurrent venous thromboembolism with increasing age. Semin Thromb Hemost 2014;40:634-9. [Crossref] [PubMed]
- Ataullakhanov FI, Koltsova EM, Balandina AN, et al. Classic and Global Hemostasis Testing in Pregnancy and during Pregnancy Complications. Semin Thromb Hemost 2016;42:696-716. [Crossref] [PubMed]
- Lippi G, Bonfanti L, Saccenti C, et al. Causes of elevated D-dimer in patients admitted to a large urban emergency department. Eur J Intern Med 2014;25:45-8. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]


