Red cell distribution width and cancer
Introduction
Red cell distribution width (RDW) is an index of the heterogeneity in the size of circulating erythrocytes and may be used to quantitate the amount of anisocytosis on peripheral blood (1). Accordingly, it reflects impaired erythropoiesis and abnormal red blood cell survival but it correlates also with inflammation, undernutrition and impaired renal function, with inadequate production of erythropoietin (EPO) (2,3).
In the last years, a number of studies have demonstrated that this simple parameter, automatically reported by laboratory blood analyzers, may have multiple clinical applications: an increased RDW has a high negative predictive value (NPV) for diagnosing a variety of disorders and may be useful to evaluate short- and long-term prognosis in cardiovascular and thrombotic disorders (4-6).
Considerable attention has been paid for the observation that RDW is a strong predictor of all-cause, cardiovascular- and cancer-related mortality in the general population (Table 1).
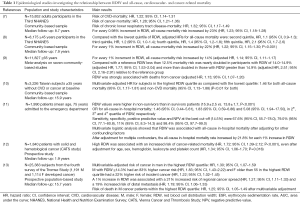
Full table
Perlstein et al. demonstrated in a community-based sample which included 15,852 adult participants in the Third National Health and Nutrition Examination Survey (NHANES) (performed between 1988 and 1994), that RDW is associated with increased mortality risk (2-fold from the lowest to the highest quintile of RDW after multivariable adjustment) and that this association is not specific to cardiovascular disease (CVD) (7). After multivariable adjustment, an increment of 0.98% in RDW resulted associated with a 23% greater risk of all-cause mortality [hazard ratio (HR), 1.23; 95% confidence interval (CI), 1.18–1.28]. Moreover, the authors observed that RDW was associated with risk of death due to CVD (HR, 1.22; 95% CI, 1.14–1.31), cancer (HR, 1.28; 95% CI, 1.21–1.36), and chronic lower respiratory tract disease (HR, 1.32; 95% CI, 1.17–1.49).
In another large epidemiological study, Patel et al. (8) measured RDW in a national sample of 8,175 community-dwelling adults 45 years or older who participated in the 1988−1994 NHANES. Interestingly, the authors demonstrated that even when analyses were restricted to non-anemic participants or to those in the reference range of RDW (11–15%) without iron, folate, or vitamin B12 deficiency, RDW remained strongly associated with mortality. In particular, compared with the lowest quintile of RDW, adjusted HR for all-cause mortality increased until 2.1 (95% CI, 1.7–2.6) in the fifth quintile. For every 1% increment in RDW, all-cause mortality risk increased by 22% (HR, 1.22; 95% CI, 1.15–1.30; P<0.001).
Successively, the same group of researcher, by performing a meta-analysis on seven community-based studies of older adults (n=11,827) confirmed that there was a graded increased risk of death associated with higher RDW values (P<0.001). Indeed, for every 1% increment in RDW, total mortality risk increased by 14% (adjusted HR, 1.14; 95% CI, 1.11–1.17). In addition, RDW was strongly associated with deaths from cancer (adjusted HR, 1.13; 95% CI, 1.07–1.20) (9).
Chen et al. enrolled in Taiwan 3,226 subjects aged 35 years or older who reported no CVD or cancer at baseline (10). During a median follow-up period of 15.9 years, 810 participants died. The multivariate-adjusted HR for subjects in the highest RDW quartile as compared with the lowest quartile was 1.46 for both all-cause mortality (95% CI, 1.17–1.81) and non-CVD mortality (95% CI, 1.13–1.88) (P<0.01 for both).
Very recently, Kim et al. demonstrated that RDW is an independent predictor of all-cause in-hospital mortality among patients older than 65 years (11). The authors investigated a total of 1,990 patients (mean age, 75 years) admitted to the emergency department with any medical problems except trauma-related injury and hematologic disease, and observed that RDW values were higher in non-survivors than in survivors patients (15.9±2.5 vs. 13.8±1.7, P<0.001). The OR for all-cause in-hospital mortality was 1.46 (95% CI, 0.44–5.61), 1.83 (95% CI, 0.59–6.86) and 5.08 (95% CI, 1.94–17.50), in 2th, 3th and 4th quartile of RDW, respectively. Sensitivity, specificity, positive predictive value and NPV at the best cut-off (14.5%) were 67.6% (95% CI, 55.7–78.0%), 79.0% (95% CI, 77.1–80.8%), 11% (95% CI, 8.3–14.3%) and 98.4% (95% CI, 97.7–99.0%).
Multivariate logistic analysis showed that RDW was associated with all-cause in-hospital mortality after adjusting for other confounding factors: the all-cause in-hospital mortality rate increased by 21.8% for each 1% increase in RDW (11).
Several studies have demonstrated that RDW is higher not only in patients affected by CVD but also in patients affected by solid tumors and hematological cancer compared to healthy individuals (12-14). As well as mortality, in these patients increased RDW has been shown to predict advanced stage and worse prognosis (15).
In a prospective, observational cohort study including 1,840 patients with solid and hematological cancer included in the prospective study named Vienna Cancer and Thrombosis Study (CATS), high RDW was found associated with an increased risk of mortality with an HR per 1% RDW increase of 1.11 (95% CI, 1.08–1.15, P<0.001). The cumulative probability of survival in patients with high RDW (>16%) was 78.5% after 6 months, 66.2% after 1 year and 41.3% after 2 years. In comparison, patients with RDW levels ≤16% had a cumulative survival probability of 88.7% after 6 months, 75.1% after one year and 66.2% after 2 years (Log-rank P<0.001) (12). The authors observed that high RDW was associated with an increased risk of cancer-related mortality (HR, 1.72; 95% CI, 1.39–2.12; P<0.001), and this association persisted after adjustment for age, sex, hemoglobin, leukocyte and platelet count (HR, 1.34; 95% CI, 1.06–1.70; P=0.016).
Ellingsen et al. performed a larger prospective population-based study by including 25,383 subjects from the fourth survey of the Tromsø Study (13,16). During the follow-up (median 15.7 years), 1,191 men and 1,114 women were diagnosed with cancer. The aim of the study was to assess the impact of RDW on future risk of incident cancer, cancer stage and mortality among cancer patients (13). The multivariable-adjusted risk of cancer resulted 30% higher in men in the highest RDW quartile compared with the lowest (HR, 1.30; 95% CI, 1.07–1.59). Moreover, men with RDW ≥14.3% had an 83% higher cancer risk (HR, 1.83; 95% CI, 1.43–2.22) and women older than 55 in the highest RDW quartile had a 22% higher risk of incident cancer than women in the three lower quartiles (HR, 1.22; 95% CI, 1.02–1.45). Interestingly, in both men and women of post-menopausal age, after multivariable adjustment, there was an association between high RDW and increased risk of regional and distal metastasis at the time of diagnosis. A 1% increase in RDW was associated with a 21% increased risk of regional cancer spread (HR, 1.21; 95% CI, 1.11–1.33) and a 19% increased risk of distal metastasis (HR, 1.19; 95% CI, 1.06–1.33). Finally, male cancer patients within the highest RDW quartile had a 25% higher risk of death during follow-up than men in the three lower quartiles (HR, 1.25, 95% CI, 1.05–1.49), after multivariable adjustment (13).
Contrarily, despite RDW resulted higher in cancer patients compared to healthy controls, Baicus et al., showed in 253 consecutive patients with involuntary weight loss admitted and followed for 6 months in a secondary care university hospital, that the performance to predict cancer was higher for other hematological indexes in comparison to RDW. The area under the curve (AUC) was 0.59 for RDW but 0.71 for C-reactive protein level, 0.69 for erythrocyte sedimentation rate (ESR), 0.65 for serum iron level, 0.61 for hemoglobin level and 0.60 for ferritin level. Moreover, in the multivariable analysis, only ESR remained associated with cancer (14).
Most of the above-mentioned studies aimed to investigate the relationship between RDW and cancer, have been performed in old populations where RDW could reflect also comorbidities such as age, risk of cardiovascular complications, and severity of renal impairment. Accordingly, it has been demonstrated that RDW increases with age (17).
Moreover, a number of studies have suggested that aging is associated with dysregulation of pro-inflammatory cytokines, in particular IL-6, which may affect erythropoiesis either by inhibition of EPO production or downregulation of EPO receptor expression (18-20).
Role of RDW in hematological tumors
Hematological tumors are known to be associated with disturbances of erythropoiesis, inflammatory microenvironments and malnutrition (21-24). Accordingly, since RDW level indicates abnormal red blood cell survival but it correlates also with the presence of severe systemic inflammatory state, undernutrition and inadequate production of EPO (3,25), this parameter has been investigated as prognostic factor and marker of disease activity in hematological malignancies [i.e., lymphocytic and myeloid leukemia, multiple myeloma, lymphoma and myelodysplastic syndrome (MDS)] (26-33).
At the end of the last century it has been demonstrated that RDW increases in hairy cell leukemia (HCL) patients and that values are related to disease activity (30). Moreover, this parameter may normalize after successful therapy (31).
Chrobák et al. (30) examined RDW in 17 patients affected by HCL treated with 2-chlorodeoxyadenosine (2-CdA), in 5 patients treated with interferon alpha (IFN-alpha) and in 9 patients subjected to splenectomy. In the first group the mean RDW value decreased from 18.8% (range, 13.5–25.0%) before therapy to 13.6% (range, 11.2–17.9%) after 6 to 12 months of successful therapy and to 13.4% (range, 12.6–14.7%) after 18 months (P=0.00015 and P=0.00049 respectively). Analogously, in the group of patients treated with IFN-alfa RDW decreased from 21.3% (range, 18.8–28.7%) to 15.3% (range, 12.4–16.7%) (P=0.031) and in 9 patients in complete hematologic remission 34 to 293 months after splenectomy the mean value of RDW was 13.9% (range, 13.0–15.5%). The authors hypothesized that the increase of RDW could be due to qualitative disturbances of erythropoiesis. Accordingly, Zák and colleagues evaluated dyserythropoietic changes in bone marrow films of 17 patients before and after therapy with 2-CdA. They observed that, after therapy, complete hematologic remission was achieved in four patients with disappearance of dyserythropoietic changes and normalization of RDW values. Contrarily, RDW remained unchanged in patient with disease progression (31).
Successively, Buckstein and colleagues developed a scoring system to predict a diagnosis of MDS in a population of patients with unexplained cytopenias and/or macrocytosis. This score included four factors: age ≥65, mean corpuscular volume (MCV) >96 fL, RDW >14.5%, and lactate dehydrogenase (LDH) >250 IU/L (32). By investigating 322 (median age, 70 years; range, 22–95 years) the probability of confirmed MDS was 70% for all four factors and ranged from 47% to 63% for three factors.
Successively, the same research group validated this simple scoring system on a population of 265 individuals (median age, 71 years; range, 20–99 years) with bone marrow examinations for unexplained cytopenia(s) and or a macrocytosis. The authors reported that the probability of the patient having the post-test diagnosis of MDS increased from 12% if they had no positive factors to 27%, 34% and 48% if they had 1, 2, 3 or 4 positive factors. Moreover, the AUC’s for the ability of the score to predict confirmed MDS confirmed or suspected MDS or confirmed or suspected MDS or AML were 0.67, 0.69 and 0.75 respectively. Sensitivity and specificity resulted 5% and 95%, 6% and 96%, 7% and 97% when four factors were positive (33).
Lee et al. longitudinally (median follow-up, 47 months) investigated 146 patients (median age, 61 years; range, 32–83 years) and demonstrated that elevated RDW at diagnosis in patients with symptomatic multiple myeloma was associated with advanced disease status and poor prognosis (26). Accordingly, patients with normal-RDW showed better progression-free survival (PFS) compared to high-RDW patients (median PFS, 24.2 vs. 17.0 months, P=0.029).
Baseline RDW level (HR, 1.69, 95% CI, 1.05–2.75, P=0.031) resulted a potential risk factor for poor PFS but resulted not prognostic for overall survival (OS) (P=0.238). Interestingly, patients who had RDW >14.5% at diagnosis were associated with higher risk of disease progression or death compared to patients with normal RDW at diagnosis (HR, 3.04; 95% CI, 1.16–8.01; P=0.024). However, in multivariate analysis for OS, RDW at diagnosis was not an independent prognostic factor (HR, 0.90, 95% CI, 0.36–2.26) after adjustment with age, performance status, cytogenetic risk group, ISS, LDH, hemoglobin, albumin, β2-microglobulin, type of treatment, and autologous stem cell transplantation.
Eighty-four newly-diagnosed chronic myeloid leukemia (CML) patients (median follow-up, 48 months; range, 3−169 months) were studied by Iriyama et al. with the aim to investigate the impact of RDW values on patient outcomes and on treatment response (28). The 5-year event-free survival (EFS) and transformation-free survival (TFS) rates were lower in high- in comparison to low-RDW group (68% vs. 100%, P=0.0071 and 81% vs. 100%, P=0.039). Moreover, CML associated deaths were observed in the high-RDW group (15%) but not in low-RDW group.
Finally, the RDW values were significantly lower 6 months after starting treatment compared to those at initial diagnosis (P<0.001) and resulted predictors of worse treatment responses by 3 and 6 months, but not by 12 months.
In agreement with these observations, RDW resulted an independent prognostic marker of poor outcome also in patients with diffuse large B cell lymphoma (DLBCL) (29). Indeed, Periša and co-authors, by investigating 81 Croatian patients (median age, 64 years) affected by DLBCL, have found RDW levels higher in patients with advanced clinical stage in comparison to early stages (14.94±1.82 vs. 13.55±1.54, P=0.001) and in those with poor response to therapy (14.94±1.82 vs. 13.55±1.54, P=0.001). Patients with RDW >15% displayed significantly worse OS (median, 33 months; range, 20−46 months) in comparison to patients with RDW <15% (median, 74 months; range, 65–82 months, P<0.001). Moreover, patients with RDW >15% had significantly worse EFS (median, 27 vs. 68 months, P<0.001). Accordingly, Cox regression analysis showed that RDW >15% was an independent prognostic factor for OS (HR, 3.66; 95% CI, 1.13–11.84) and EFS (HR, 2.61; 95% CI, 1.012–6.739).
Interestingly, the authors observed a significant association not only between RDW and CRP but also between RDW and hypoalbuminemia, which is indicative of malnutrition and mortality (29).
Recently, Podhorecka et al., with the aim to investigate RDW as a marker of prognosis in patients with chronic lymphocytic leukemia (CLL), enrolled 66 previously untreated persons (median age, 63 years; range, 38–85 years) with CLL diagnosis (27). They reported that RDW is in correlation with prognostic factors such as clinical stadium of the disease and expression of ZAP-70 and CD38, both indicators for immunoglobulin heavy chain genes mutations. The differences in time to treatment and in OS between low-RDW group and high-RDW group were observed, however they were not statistically significant. In multivariate Cox proportional hazard regression analysis including RDW, CD38 expression, ZAP-70 expression and group of cytogenetic risk, the RDW level and ZAP-70 expression were found to be the independent predictors of shorter survival (P=0.04 and P=0.03, respectively). However, by longitudinally evaluating RDW values (at diagnosis, during disease progression and at the end of chemotherapy), there was no significant differences in RDW as the disease progress. Thus, the authors concluded that RDW is stable, not time-dependent prognostic marker.
Role of RDW in solid cancers
A growing body of evidence has suggested that RDW might have a role as diagnostic or prognostic marker in various solid cancers. In particular, most published studies focused on RDW at the time of diagnosis as an independent and reproducible predictor of cancer patient survival.
To date, five studies have investigated the prognostic value of RDW in esophageal cancer (34-38). The study characteristics and the main findings of these studies are reported in Table 2. All studies were retrospective, single institution design studies performed in China or Japan with overall good sample size and almost quite long follow-up period. All studies evaluated the prognostic value of preoperative RDW levels since all patients underwent potentially curative resection in association or not with radio and/or chemotherapy. Four out of five studies enrolled patients affected by esophageal squamous cell carcinoma (ESCC). The remaining one included patients with either squamous or adenocarcinoma subtype (35). All studies applied similar cut-off values ranging from 12.2% to 15.3% for dividing patients in high and low RDW categories. Unfortunately, only the groups of Sun P. and Chen GP., determined the optimal cut-off value for RDW by receiver operating characteristic (ROC) curves (34,38) while the others set the values on the upper limit of reference range used in routine laboratory analyses thus prompting caution in the interpretation of data.
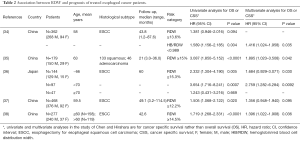
Full table
In univariate analyses RDW always emerged as significant risk factors for a poor prognosis with HR ranging from 1.381 to 3.087 for OS and from 1.719 to 2.332 for CSS. Wan et al. (35) and Zhang et al. (37) also found a significant association between high RDW and shorter DFS (HR, 3.208; 95% CI, 1.922−5.353; P<0.0001 and 1.474; 95% CI, 1.046−2.077; P=0.027 respectively). Moreover, the results of the multivariate analyses overall qualified RDW as independently predictor of patient OS or cancer specific survival (CCS) survival. However, Zhang et al. reported that RDW was no longer associated with either OS or DFS after adjustment for age, lymph node metastasis, TNM stage, adjuvant therapy, smoking status, maximum tumor diameter, MPV, PLT, CA19-9, NLR (Neutrophil-lymphocyte ratio) and COP-MPV (combination of platelet count and MPV). Notably, in the study of Sun (34), while the crude RDW showed no significant association with OS the combination of RDW and HB values in the form of hemoglobin/red blood cell distribution width (HB/RDW) ratio was found independently associated with OS even after adjusting for lymph node status, tumor depth, treatment, tumor size and the Glasgow prognostic score (GPS). Interesting, Hirahara and colleagues (36) found that a high RDW is potentially an independent risk factor for a worse prognosis in non-elderly patients, but not in elderly patients. The authors hypnotized that the higher prevalence of anemia and malnutrition in the latter group, by leading to elevated RDW values, could in turn reduce its prognostic significance for cancer.
Studies evaluating the prognostic role of RDW in solid cancers different from esophageal cancer are still limited although some other evidence can arise from studies on lung and breast cancers.
As shown in Table 3 studies performing univariate and multivariate Cox proportional hazard model for significance of prognostic variables on patients with lung cancer consistently demonstrated that RDW is a significant factor after risk adjustment, determining long-term survival. Overall, such association has been found independently of tumor histological subtype. However, while in small cell lung cancer (SCLC), RDW seems to have a major role in extensive stage with respect to limited stage (39), in non-small cell lung cancer (NSCLC) the association between high RDW and poor prognosis has been consistently revealed in any stage (40). In a large-size single center retrospective study, Warwick and coauthors demonstrated that RDW is a significant determinant not only of OS but also of length of hospital stay, in-hospital morbidity, superficial thoracotomy wound infections need for postoperative respiratory support and in-hospital mortality post-potentially curative resections for non-small-cell lung cancer (41).

Full table
Studies investigating the prognostic role of RDW in patients with breast cancer are described in Table 4. Even in this type of solid cancer, available evidences suggest that an elevated pretreatment RDW is an independent factor of poor survival in women with breast cancer. The association between RDW and worse prognosis was found for both young women (42) and women over 50 years (43,44) and seemed to be stronger for white women than for black ones (43).

Full table
In one of these studies high pretreatment RDW, positive PR status, more advanced stage, and PVI presentation were also independent prognostic factors for DFS (42). In particular, the 5-years DFS rate of patients in the high RDW group was 58.44% while those in the low RDW group was 91.78% (P<0.0001).
A part from the role of RDW as prognostic biomarker in solid cancers some data also exist concerning the potential consideration of RDW as a biomarker of cancer diagnosis, growth and metastatic activity.
According to two independent studies, RDW has been reported to be a useful biomarker to distinguish between benign or malignant breast tumors. Moreover, RDW elevation was significantly correlated with larger primary tumors, higher number of infiltrated axillary lymph nodes, and advanced stages (42,45).
A recent study by Beyazit et al. indicated that elevated RDW could be a useful biomarker in order to discriminate benign from malignant causes of biliary obstruction, with a sensitivity of 72% and specificity of 69%, using a cut-off value of 14.8% (46). Similarity, Wang and colleagues demonstrated that high RDW value (12.85%) could predict the presence of renal cell carcinoma (RCC). In addition, the data revealed a positive association between RCC stage, grade, and the level of RDW and also determined the cut-off points (13.15%) of RDW which can be valuable for predicting advanced RCC (47).
Three other studies assessing the utility of RDW as an additional factor for increasing the diagnostic accuracy of anemia as a screening method in colorectal cancer demonstrated, instead, conflicting results. Spell et al. reported that RDW could be a useful parameter in predicting right-sided colon cancer with a sensitivity of 84% and specificity of 88%. This result might be due to iron deficiency (48). Ay et al., also suggested that RDW can be used as an early warning biomarker for solid colon tumors since RDW values of patients with colon cancer were significantly higher than the patients with colon polyp (P=0.01) (49). In contrast, Speights et al. found that the addition of MCV and RDW to the hemoglobin value does not seem to increase the sensitivity of the CBC in the detection or clinical suspicion of colorectal carcinoma (50).
In a recent study Kemal et al., by investigating the potential predictive role of RDW in 884 patients with post-menopausal bleeding found that RDW was significantly higher in patients with endometrial cancer (EC) with respect to patients in the benign group (14.78 ± 2.02 vs. 13.88 ± 1.05; P=0.000) (51). RDW has been found significantly increased also in patients with primary hepatocellular carcinoma (HCC) where it correlated with liver function tests (52) and was associated with poor survival (53). A strong correlation between RDW levels and disease prognosis and stages was demonstrated also for disseminated solid malignancies to the bone marrow (54,55) and pancreatic cancer (56).
Conclusions
Overall such studies confirm the existence of a statistically significant association between RDW and increased solid and hematological cancer risk. However, the fact that, after adjustment for other hematological and inflammatory parameters, RDW often resulted no longer associated with cancer risk and mortality, seems to support the explanation according to which, the link between RDW and cancer simply reflects the role of RDW in inflammation and oxidative stress which are, in fact, risk factors for cancer (57).
Further prospective and larger studies are required to establish the role of RDW as early biomarker for cancer diagnosis or activity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morris M, Davey FR. Basic examination of blood. In: Henry JB, editor. Clinical diagnosis and management by laboratory methods. 20th Edition. Philadelphia: W.B. Saunders Company, 2001.
- Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659-66. [Crossref] [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 2015;7:E402-11. [PubMed]
- Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 2011;50:635-41. [PubMed]
- Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588-94. [Crossref] [PubMed]
- Patel KV, Ferrucci L, Ershler WB, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med 2009;169:515-23. [Crossref] [PubMed]
- Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 2010;65:258-65. [Crossref] [PubMed]
- Chen PC, Sung FC, Chien KL, et al. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol 2010;171:214-20. [Crossref] [PubMed]
- Kim SH, Yeon JH, Park KN, et al. The association of Red cell distribution width and in-hospital mortality in older adults admitted to the emergency department. Scand J Trauma Resusc Emerg Med 2016;24:81. [Crossref] [PubMed]
- Riedl J, Posch F, Königsbrügge O, et al. Red cell distribution width and other red blood cell parameters in patients with cancer: association with risk of venous thromboembolism and mortality. PLoS One 2014;9:e111440. [Crossref] [PubMed]
- Ellingsen TS, Lappegård J, Skjelbakken T, et al. Impact of red cell distribution width on future risk of cancer and all-cause mortality among cancer patients - the Tromsø Study. Haematologica 2015;100:e387-9. [Crossref] [PubMed]
- Baicus C, Caraiola S, Rimbas M, et al. Utility of routine hematological and inflammation parameters for the diagnosis of cancer in involuntary weight loss. J Investig Med 2011;59:951-5. [Crossref] [PubMed]
- Patel HH, Patel HR, Higgins JM. Modulation of red blood cell population dynamics is a fundamental homeostatic response to disease. Am J Hematol 2015;90:422-8. [Crossref] [PubMed]
- Jacobsen BK, Eggen AE, Mathiesen EB, et al. Cohort profile: the Tromso Study. Int J Epidemiol 2012;41:961-7. [Crossref] [PubMed]
- Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med 2015;53:2015-9. [Crossref] [PubMed]
- Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood 2005;105:2294-9. [Crossref] [PubMed]
- Ble A, Fink JC, Woodman RC, et al. Renal function, erythropoietin, and anemia of older persons: the InCHIANTI study. Arch Intern Med 2005;165:2222-7. [Crossref] [PubMed]
- Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med 2005;118:1288. [Crossref] [PubMed]
- Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res 2013;37:214-20. [Crossref] [PubMed]
- Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med 1997;336:1657-64. [Crossref] [PubMed]
- Koschmieder S, Mughal TI, Hasselbalch HC, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia 2016;30:1018-24. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Ershler WB, Sheng S, McKelvey J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc 2005;53:1360-5. [Crossref] [PubMed]
- Lee H, Kong SY, Sohn JY, et al. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int 2014;2014:145619.
- Podhorecka M, Halicka D, Szymczyk A, et al. Assessment of red blood cell distribution width as a prognostic marker in chronic lymphocytic leukemia. Oncotarget 2016;7:32846-53. [PubMed]
- Iriyama N, Hatta Y, Kobayashi S, et al. Higher Red Blood Cell Distribution Width Is an Adverse Prognostic Factor in Chronic-phase Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors. Anticancer Res 2015;35:5473-8. [PubMed]
- Periša V, Zibar L, Sinčić-Petričević J, et al. Red blood cell distribution width as a simple negative prognostic factor in patients with diffuse large B-cell lymphoma: a retrospective study. Croat Med J 2015;56:334-43. [Crossref] [PubMed]
- Chrobák L, Zák P, Podzimek K, et al. Red cell distribution width (RDW) as a marker of disease activity in patients with hairy cell leukemia. Acta Medica (Hradec Kralove) 1998;41:23-6. [PubMed]
- Zák P, Chrobák L, Podzimek K, et al. Dyserythropoietic changes and sideroblastic anemia in patients with hairy cell leukemia before and after therapy with 2-chlorodeoxyadenosine. Neoplasma 1998;45:261-5. [PubMed]
- Buckstein R, Jang K, Friedlich J, et al. Estimating the prevalence of myelodysplastic syndromes in patients with unexplained cytopenias: a retrospective study of 322 bone marrows. Leuk Res 2009;33:1313-8. [Crossref] [PubMed]
- Rauw J, Wells RA, Chesney A, et al. Validation of a scoring system to establish the probability of myelodysplastic syndrome in patients with unexplained cytopenias or macrocytosis. Leuk Res 2011;35:1335-8. [Crossref] [PubMed]
- Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern china. Oncotarget 2016. [Epub ahead of print]. [PubMed]
- Wan GX, Chen P, Cai XJ, et al. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta 2016;452:199-203. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Kawahara D, et al. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol 2016;21:909-19. [Crossref] [PubMed]
- Zhang F, Chen Z, Wang P, et al. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol 2016;37:9323-31. [Crossref] [PubMed]
- Chen GP, Huang Y, Yang X, et al. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediators Inflamm 2015;2015:854670.
- Xie D, Marks R, Zhang M, et al. Nomograms Predict Overall Survival for Patients with Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. J Thorac Oncol 2015;10:1213-20. [Crossref] [PubMed]
- Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. [Crossref] [PubMed]
- Warwick R, Mediratta N, Shackcloth M, et al. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:108-13. [Crossref] [PubMed]
- Huang DP, Ma RM, Xiang YQ. Utility of Red Cell Distribution Width as a Prognostic Factor in Young Breast Cancer Patients. Medicine (Baltimore) 2016;95:e3430. [Crossref] [PubMed]
- Wang C, Civan J, Lai Y, et al. Racial disparity in breast cancer survival: the impact of pre-treatment hematologic variables. Cancer Causes Control 2015;26:45-56. [Crossref] [PubMed]
- Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther 2014;7:1743-52. [PubMed]
- Seretis C, Seretis F, Lagoudianakis E, et al. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J Clin Med Res 2013;5:121-6. [PubMed]
- Beyazit Y, Kekilli M, Ibis M, et al. Can red cell distribution width help to discriminate benign from malignant biliary obstruction? A retrospective single center analysis. Hepatogastroenterology 2012;59:1469-73. [PubMed]
- Wang FM, Xu G, Zhang Y, et al. Red cell distribution width is associated with presence, stage, and grade in patients with renal cell carcinoma. Dis Markers 2014;2014:860419.
- Spell DW, Jones DV Jr, Harper WF, et al. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev 2004;28:37-42. [Crossref] [PubMed]
- Ay S, Eryilmaz MA, Aksoy N, et al. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pac J Cancer Prev 2015;16:753-6. [Crossref] [PubMed]
- Speights VO, Johnson MW, Stoltenberg PH, et al. Complete blood count indices in colorectal carcinoma. Arch Pathol Lab Med 1992;116:258-60. [PubMed]
- Kemal Y, Demirag G, Baş B, et al. The value of red blood cell distribution width in endometrial cancer. Clin Chem Lab Med 2015;53:823-7. [Crossref] [PubMed]
- Wei TT, Tang QQ, Qin BD, et al. Elevated red blood cell distribution width is associated with liver function tests in patients with primary hepatocellular carcinoma. Clin Hemorheol Microcirc 2016. [Epub ahead of print]. [PubMed]
- Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark 2016;16:507-12. [Crossref] [PubMed]
- Ozkalemkas F, Ali R, Ozkocaman V, et al. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer 2005;5:144. [Crossref] [PubMed]
- Seitanides B, Giakoumakis G, Tsakona C. Increased red cell volume distribution width in patients with bone marrow metastases. J Clin Pathol 1988;41:1246. [Crossref] [PubMed]
- Yilmaz A, Malya F, Ozturk G, et al. Effect of pre-operative red blood cell distribution on cancer stage and morbidity rate in patients with pancreatic cancer. Int J Clin Exp Med 2014;7:3072-5. [PubMed]
- Means RT Jr. Higher red blood cell distribution width was associated with increased risk of mortality in adults > or =45 years of age. Evid Based Med 2009;14:151. [Crossref] [PubMed]


