
Hereditary spherocytosis complicated by intrahepatic cholestasis: two case reports
Introduction
Hereditary spherocytosis (HS) is a chronic hemolytic disease caused by congenital hereditary red cell membrane defects, and its inheritance pattern is mostly autosomal dominant (1). Due to the mutation of the erythrocyte membrane encoding genes, the synthesis of skeleton protein is hindered, and the lipids of the erythrocyte membrane are lost, which eventually leads to a reduction in the cell surface area and a change in the spherical shape. This spherical transformation increases cell permeability, secondary to the imbalance of the sodium-potassium pump, which leads to intracellular sodium retention and further induces the spherocytic shape of erythrocytes (2). Abnormal red blood cells (RBCs) remain in the splenic cord in large numbers and are eventually destroyed and dissolved. The typical clinical manifestations of HS are intermittent hemolytic anemia, splenomegaly, and increased bilirubin (mostly indirect bilirubin). A peripheral blood smear will show increased spherocytosis, and laboratory tests will show an increased osmotic fragility (OF) value for the RBC (3).
In the Nordic region, the incidence of HS is 1/5,000–1/2,000, while in China, the incidence of HS has not yet been reported (4). According to a study based on a disease model, the prevalence of HS in China is 1.27 cases per 100,000 males and 1.49 cases per 100,000 women (5). HS is not a rare disease in the department of hematology, but due to the varying severity of symptoms, typical symptoms often do not appear at the same time, and most patients are referred to the department of hepatology with “unexplained jaundice”. Early-stage HS caused hemolytic jaundice, and cholestatic jaundice and hepatocellular jaundice may develop as the disease progresses. Therefore, it is very challenging for hepatologists to make a differential diagnosis
This article reports 2 patients with “jaundice of [an] unknown origin” treated in our hospital. The clinical manifestations were similar, and they were finally diagnosed with HS due to new mutations in HS-related genes by genetic testing. However, the prognosis was different due to different treatments. We describe their clinical history and summarize the diagnostic and therapeutic procedures used in the cases to provide some insights for hepatologists into how to manage such patients. The clinical course timeline of the 2 patients is shown in Figure 1. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5076/rc).
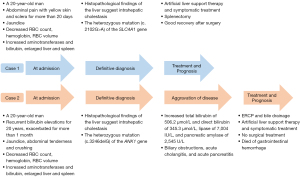
Case presentation
All the procedures performed in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from each patient or patient’s families for publication of these case reports and accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Patient 1
A 20-year-old male was transferred to our hospital suffering from “abdominal pain with yellow skin and sclera for more than 20 days”. The abdominal pain was accompanied by elevated amylase and lipase, and the patient had been diagnosed with acute pancreatitis at a local hospital and given symptomatic treatment. The patient had no history of alcohol abuse or specific drug use, and none of his family members had suffered from a similar illness.
The physical examination revealed moderate jaundice of the skin and sclera, and a palpable spleen 5 cm below the ribs, but no other abnormal findings. The routine blood tests of our emergency department showed that the RBC count was 3.59×1012/L (reference value: 4.3×1012–5.8×1012/L), the hemoglobin was 11.6 g/L (reference value: 130–175 g/L), and the RBC volume was 0.34 L/L (reference value: 0.40–0.50 L/L). Blood biochemistry indicated elevated liver enzymes, with alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT) of 622, 232, 219, and 386 IU/L, respectively. The level of bilirubin was also significantly increased. The total bilirubin was 165 µmol/L (reference value: 5–28 µmol/L), of which direct bilirubin and indirect bilirubin were 86.7 µmol/L (reference value: <8.8 µmol/L) and 78.3 µmol/L (reference value: <20 µmol/L), respectively.
After receiving basic supportive treatment, the patient was admitted to our department with “liver function abnormalities of unknown origin”. The results of the repeat routine blood tests showed mild anemia and were as follows: RBC count: 2.83×1012/L (reference value: 4.3×1012–5.8×1012/L), hemoglobin: 92 g/L (reference value: 130–175 g/L), hematocrit: 0.27 L/L (reference value: 0.40–0.50 L/L), and his reticulocyte (Ret) count was elevated to 0.464×1012/L (reference value: 0.024–0.084×1012/L). The incubated OF value of the RBC was 4.6 g/L (reference value: 4.0–4.5 g/L), which was mildly abnormal. The peripheral blood erythrocyte morphology test suggested that the percentage of the small RBC was elevated at 26.9%, with a reference value of <11%. The Coombs test was negative.
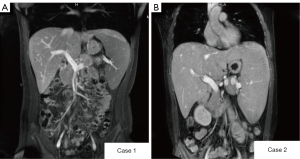
A magnetic resonance imaging (MRI) enhancement scan of the upper abdomen suggested increased liver volume and heterogeneous enhancement in the arterial phase, which suggested liver parenchymal damage, enlarged spleen volume with iron overload, and possible gallbladder stones (Figure 2). The results of the remaining examinations were normal, including the virology-related examination, immune-related disease marker, and tumor and metabolism-related marker results.
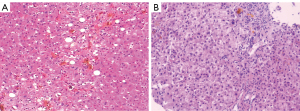
A liver biopsy was performed because the patient had liver damage with an unknown cause. The histopathology of the liver revealed intrahepatocellular biliary stasis with visible bile embolus inside capillary bile ducts, which were partially dislodged in the hepatic sinusoids, accompanied by Kupffer cell proliferation, and mixed steatosis (25%) in the surrounding hepatocytes. Neutrophil infiltration was observed in the portal duct area, and cytokeratin 7 (CK7) showed mild hyperplasia of small bile ducts, with bile embolus formation in some dilatated bile ducts. Prussian blue staining showed a mild increase in iron content in hepatocytes, and the diagnosis of cholestatic liver injury with intrahepatocellular cholestasis in the glandular alveolus III region was considered (Figure 3); however, based on the previous examination results, the diagnosis was not confirmed.
The patient had chronic hemolytic anemia. Thus, whole-exome gene sequencing was performed to elucidate the etiology of anemia. The results suggested that a heterozygous missense mutation (c.2102G>A) occurred in the solute carrier family 4, member 1 (SLC4A1) gene located on chromosome 17. The SLC4A1 mutation is one of the causes of HS (Figure 4). At this point, the diagnosis of HS was clear.

After artificial liver support therapy and symptomatic treatment, the patient’s bilirubin decreased to 125.4 µmol/L, his albumin and coagulation function indexes were normal, and a splenectomy was performed at the local hospital. After the operation, the patient’s liver function and routine blood results quickly returned to normal, and the liver function was also normal during the follow-up. The changes in the laboratory test items during the patient’s hospitalization are shown in Table 1.
Table 1
| Days after admission | Total bilirubin (μmol/L) | Direct bilirubin (μmol/L) | Direct bilirubin ratio | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | GGT (IU/L) | RBC (×1012/L) |
Hematocrit (L/L) | Red cell distribution width (fL) | Hemoglobin (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 165.0 | 86.7 | 52.6% | 622 | 232 | 219 | 386 | 3.59 | 0.34 | 53.6 | 116 |
| 4 | 136.0 | 73.5 | 54.0% | 415 | 104 | 166 | 303 | 3.28 | 0.31 | 54.7 | 108 |
| 7 | 143.8 | 67.3 | 46.8% | 168 | 59 | 129 | 186 | 2.83 | 0.27 | 62.8 | 92 |
| 11 | 118.5 | 56.0 | 47.3% | 174 | 74 | 113 | 143 | 2.92 | 0.28 | 67.8 | 96 |
| 14 | 125.4 | 48.9 | 39.0% | 117 | 47 | 107 | 113 | 3.03 | 0.30 | 71.2 | 101 |
| 6 months (after splenectomy) | 22.3 | 5.2 | 23.3% | 30 | 24 | 52 | 35 | 4.30 | 0.40 | 54.0 | 130 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; RBC, red blood cell.
Patient 2
A 20-year-old young man was admitted to our hospital with “recurrent skin sclera yellowing for 20 years, exacerbated for more than 1 month”. The patient had developed anemia of unknown origin after vaccination at 3 months old. Since then, he had suffered from intermittent skin jaundice and scleral icterus, mostly after a cold.
The physical examination showed that the patient’s skin and sclera were yellow, and the abdominal tenderness was scattered, with the strongest tenderness under the subxiphoid. The routine blood results showed that his RBC count was 3.96×1012/L, his Ret count was 0.38×1012/L, his hemoglobin was 128 g/L, and his hematocrit was 0.37 L/L. The blood smear results showed that 22.9% of the RBC were of different sizes (reference value 11%), with an increase in the small RBC of 42.2% (reference value 11%), visible cleaved RBC (1%), and spherical RBC (7%). The OF test of the RBC suggested mild elevation (5.9 g/L; reference value 4–4.5 g/L). His haptoglobin was <58.3 mg/L, and thus significantly decreased (reference value 500–2,200 mg/L). The results of the Coombs test was negative. The haptoglobin in the serum had decreased significantly and was lower than the detection limit (58.3 mg/L). His serum ferritin level was elevated to 344 ng/mL. His bilirubin was also considerably increased, with a total bilirubin of 954.4 µmol/L, direct bilirubin of 607.6 µmol/L, and indirect bilirubin of 346.8 µmol/L. There were no significant abnormalities in the liver enzymes except for an increased GGT of 185 IU/L. His total protein had decreased to 49.7 g/L (reference value: 68–108 g/L), his globulin had decreased significantly to 8.4 g/L (reference value 20–40 g/L), and the A/G ratio had increased to 4.92.
The virologic examination results showed the patient was Epstein-Barr-virus positive but <50 copies/mL. The results of the other tests were normal, such as the coagulation-related indicator, virology-related marker, immune disease marker, tumor biomarker, and metabolism-related marker results. A MRI scan of the upper abdomen showed an enlarged liver and spleen, portal hypertension, iron deposition in the spleen, hemangioma of the left lobe of the liver, and multiple gallbladder stones with cholecystitis (Figure 2).
We suspected he had HS, and genetic testing was performed to confirm the diagnosis. The gene sequencing results suggested a heterozygous mutation (c.3246delG) in the ankyrin 1 (ANK1) gene, which may be associated with HS (Figure 4). In addition, the results also showed that there were heterozygous mutations in some genes, including uridine diphosphate glucuronosyltransferase family 1 member A1 (UGT1A1), mannosidase alpha class 2B member 1 (MAN2B1), and acyl-CoA dehydrogenase short/branched chain (ACADSB), but only when homozygous mutation in these genes can cause inherited metabolic liver disease.
A liver biopsy was also performed to clarify the cause of the liver injury. The pathological findings suggested visible hepatocyte regeneration, scattered punctate, and focal necrosis in the lobules, extensive hepatocellular siltation with bile embolus formation, and mild to moderate interface inflammation. Many lymphocytes, monocytes, a few plasma cells, and scattered neutrophils were observed in the portal duct area. The positive staining of CK7 showed obvious hyperplasia of the small bile ducts. Foot and Masson staining showed fibrous tissue hyperplasia and the portal duct area enlargement with a fibrous septum, partially separating the liver lobules (Figure 3).
Due to the multiple stones in his gallbladder, we sought the help of hepatobiliary surgeons to perform a cholecystectomy. However, the hepatobiliary surgeon considered that his liver function could not tolerate the surgery, which was extremely risky to perform. Thus, he was given glucocorticoid anti-inflammatory therapy, sucralfate to protect the gastric mucosa, and continued liver protection therapy and jaundice reduction therapy.
The patient’s symptoms improved. His total bilirubin decreased to 267.1 µmol/L, and his direct bilirubin decreased to 197.3 µmol/L. Unfortunately, on the 30th day of admission, the patient experienced severe right upper quadrant pain with increased total bilirubin of 506.2 µmol/L and direct bilirubin of 345.3 µmol/L, lipase of 7,004 IU/L, and a pancreatic amylase of 2,545 U/L. Enhanced computed tomography of the whole abdomen suggested an enlarged liver and spleen, a thickened portal vein and splenic vein, intrahepatic lymphatic stasis, hypodensity of the liver, multiple gallbladder stones, a thickened and gross gallbladder wall, chronic cholecystitis, peripancreatic exudative changes, scattered small amounts of fluid in the pelvic and abdominal cavities, and signs of peritonitis. Thus, he was diagnosed with biliary obstructions, acute cholangitis, and acute pancreatitis.
Endoscopic retrograde cholangiopancreatography (ERCP) was performed to clear the biliary tract, and an endoscopic nasociliary drainage tube was placed for bile drainage. The patient’s abdominal pain was slightly relieved, but his bilirubin level increased. Given that the patient had HS and multiple gallbladder stones, cholecystectomy was recommended to prevent the gallstones from falling into the biliary tract again and aggravating the disease. However, the patient’s current bilirubin level was still very high, and the surgical risk was extremely high. Unfortunately, the patient eventually stopped treatment and passed away 2 weeks after leaving the hospital. The changes in the laboratory test item results during the patient’s hospitalization are shown in Table 2.
Table 2
| Days after admission | Total bilirubin (μmol/L) | Direct bilirubin (μmol/L) | Direct bilirubin ratio | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | GGT (IU/L) | RBC (×1012/L) | Hematocrit (L/L) |
Red cell distribution width (fL) | Hemoglobin (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 474.4 | 327.4 | 69.0% | 80 | 43 | 166 | 223 | 3.96 | 0.37 | 67.9 | 128 |
| 3 | 774.3 | 506.3 | 65.4% | 58 | 36 | 165 | 253 | 3.40 | 0.32 | 66.3 | 109 |
| 5 | 954.4 | 607.6 | 63.7% | 43 | 48 | 145 | 185 | 3.30 | 0.30 | 64.9 | 105 |
| 9 | 557.2 | 442 | 79.3% | 30 | 32 | 144 | 146 | 3.14 | 0.30 | 67.1 | 102 |
| 12 | 499.2 | 387.8 | 77.7% | 21 | 30 | 133 | 118 | 3.11 | 0.29 | 62.4 | 100 |
| 30 | 267.1 | 197.3 | 73.9% | 21 | 30 | 133 | 118 | 3.99 | 0.37 | 65.1 | 131 |
| 31 | 387.8 | 243.6 | 62.8% | 31 | 32 | 132 | 283 | 3.51 | 0.33 | 64.5 | 115 |
| 33 | 506.2 | 345.3 | 68.2% | 60 | 57 | 147 | 365 | 2.97 | 0.28 | 67.1 | 94 |
| 35 | 733.2 | 538.9 | 73.5% | 23 | 26 | 95 | 91 | 2.78 | 0.26 | 64.8 | 91 |
| 40 (discharged) | 896.6 | 772.9 | 86.2% | 29 | 36 | 122 | 61 | 2.87 | 0.27 | 63.1 | 94 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; RBC, red blood cells.
Discussion
HS is mainly caused by defects in 1 or more of the 5 RBC membrane proteins, including erythrocyte membrane protein band 3, erythrocyte membrane protein band 4.2 (EPB42), ankyrin, α-spectrin (SPTA1), and β-spectrin (SPTB), and is mainly an autosomal dominant genetic disease (1,2). The main clinical manifestations are hemolytic anemia, jaundice, and splenomegaly, and some patients may also suffer from gallstones at the late stage. The diagnosis of HS mainly relies on typical clinical symptoms and relevant laboratory tests, such as increased free bilirubin, increased reticulocytes, peripheral blood smear with a spherical RBC >7–10%, and an increased RBC OF value (6). The diagnosis can be confirmed by a positive family history or gene sequencing that reveals mutations in target genes encoding membrane proteins, including SPTA1, SPTB, ANK1, SLC4A1, and EPB42 (7).
Both patients in this article underwent next-generation sequencing, and novel mutation sites were found in the causative gene of HS in both patients. Patient 1 had a c.2102G>A heterozygous mutation in the SLC4A1 gene, and Patient 2 had a c.246delG mutation in the ANK1 gene, neither of which had been previously reported. Currently, there are no specific treatments for the underlying RBC membrane defects. In addition to conventional symptomatic treatment, splenectomy is feasible for relatively severe anemia and bilirubin >34 µmol/L (8).
As Patient 1 presented mainly with jaundice, splenomegaly, anemia, elevated reticulocytes, positive RBC OF test results, and negative Coombs test results, the possibility of HS was considered. However, his bilirubin was dominated by elevated direct bilirubin levels and was considered a manifestation of cholestasis but was not consistent with HS. A liver biopsy was performed to clarify the diagnosis, and the results suggested that he had a clear cholestatic presentation. In addition, gene sequencing was performed, and a mutation was found in the SLC4A1 gene. The patient was thus diagnosed HS.
Intrahepatic cholestasis might be secondary to long-term HS, but it is difficult to determine whether other factors cause it. A case with a similar clinical presentation was previously reported. In that case, the patient was ultimately considered to have a transient cholestasis manifestation due to poor bile drainage, and the cholestasis manifestation improved significantly after ERCP treatment (9). Patient 1 suffered from abdominal pain, and a MRI of the abdomen also suggested the possibility of gallstones. However, as no dilatation of the intrahepatic and extrahepatic bile ducts was observed on the MRI scan, his intrahepatic cholestasis could not be explained by simple biliary obstruction. After treatment, his direct bilirubin level gradually decreased, and his indirect bilirubin stabilized at high levels. Our findings suggest that when a hepatologist encounters a patient with cholestasis that coexists with anemia and splenomegaly, relevant tests for hemolysis should be performed and changes in bilirubin should be dynamically observed, and a diagnosis made after careful consideration.
Patient 2 showed signs of hemolysis at a young age, mostly aggravated by infection, but was never formally diagnosed and treated. The diagnosis of HS was confirmed based on his clinical symptoms and next-generation sequencing results. However, HS patients generally have predominantly elevated indirect bilirubin levels, and their total bilirubin is usually <85 µmol/L. Patient 2 had a significant elevation of direct bilirubin on admission, accounting for 63.67% of the total bilirubin, and a total bilirubin as high as 954.4 µmol/L. This could not be explained by HS alone, nor was it consistent with the performance of acute hemolytic crisis.
MRI suggested multiple gallbladder stones with cholecystitis, which is compatible with the complication of gallbladder stones in patients with severe HS, which may also be directly associated with a significant increase in direct bilirubin. The recurrent abdominal pain may be related to biliary obstruction, but his bilirubin levels still showed an upward trend after ERCP treatment, which could not be explained by the extrahepatic biliary obstruction alone. The liver biopsy showed significant cholestasis in the hepatocytes and bile capillaries; however, it was unclear how the intrahepatic cholestasis had developed. We summarized similar case reports of HS with intrahepatic cholestasis (Table 3).
Table 3
| Author | Year | No. of patients | Age (years), gender | Mutation gene and sites | Liver biopsy results | Treatment | Total bilirubin levels before and after surgery | Clinical outcome |
|---|---|---|---|---|---|---|---|---|
| Zhao (10) | 2010 | Patient 1, son | 23, male | No mutation was found in ANK1, BAND3, or ATP8 | Diffuse capillary cholestasis | Splenectomy | Before: 443.7 μmol/L | Survival |
| After: 65 μmol/L | ||||||||
| Patient 2, father | 48, male | Iron deposition in hepatocytes | Splenectomy | Before: 183.1 μmol/L | Survival | |||
| After: 26.7 μmol/L | ||||||||
| Wree (11) | 2011 | 1 | 18, male | Heterozygous variants of ABCB11 A444V and 3084A>G | Severe hepatic and mild canalicular cholestasis | Ursodeoxycholic acid and intermittent therapy with prednisone | – | Survival |
| Zhou (12) | 2015 | 1 | 49, female | Single nucleotide polymorphisms in ATP8B1 and ABCB11 | Hepatic and mild canalicular cholestasis | Splenectomy and cholecystectomy | Before: 178.3 μmol/L | Survival |
| After: 37.9 μmol/L | ||||||||
| Kalinke (9) | 2013 | 1 | 28, male | Not mentioned | Evidence of cholestasis within hepatocytes but no canalicular cholestasis | ERCP and cholecystectomy | Before ERCP: 309 μmol/L | Survival |
| After cholecystectomy: 97 μmol/L | ||||||||
| Zhang (13) | 2019 | 1 | 5, boy | SPTB, c.1791delG | Severe hepatic and mild canalicular cholestasis | Symptomatic treatments | – | Survival |
HS, hereditary spherocytosis; ERCP, endoscopic retrograde cholangiopancreatography.
Jaundice is a symptom or sign of severe hyperbilirubinemia. Common types of jaundice include hemolytic jaundice, hepatocellular jaundice, and cholestatic jaundice. For physicians, the ability to make a differential diagnosis of jaundice is a basic skill required for clinical work. The 2 patients discussed in this article had different manifestations of these 3 types of jaundice at varying stages of the disease course. Hemolytic jaundice is caused by HS in the early stage, and cholestatic jaundice is caused by intrahepatic cholestasis in the later stage, and hepatocellular injury may be present throughout disease progression.
The mechanism of HS with severe intrahepatic cholestasis has not yet been fully elucidated, and we discussed the possible mechanisms. Hepatocytes play an important role in bilirubin metabolism, including the 3 processes related to the uptake, binding, and excretion of bilirubin, which involve numerous enzymes and ion pumps (14,15). When hypersplenism occurs after long-term HS, many damaged RBCs enter the bloodstream, the enzymatic or ion-pump activity may be insufficient, and the normal physiological balance may be disrupted, thus causing an immunoinflammatory response in the liver tissue. Zhao et al. (10) reported the case of a similar patient, and they also considered that the intrahepatic cholestasis was secondary to HS with hypersplenism, and excess damaged RBCs entered the liver to induce an excessive immunoinflammatory response. It has been suggested that when HS patients present with hyperbilirubinemia, their hemolytic symptoms may be temporarily relieved, which may be related to abnormal lipid and cholesterol metabolism, but the exact mechanism remains to be investigated (16). Both patients in this article also had cholestasis as the main clinical manifestation, while their anemia and hemolysis symptoms were not severe.
Zhou et al. (12) suggested that severe intestinal infections and biliary and peripheral inflammation-inducing severe cholestasis inconsistent with a compensated state of liver function are common in clinical practice. These patients usually present with rapidly deepening jaundice after an episode of abdominal pain. However, the levels of other liver function parameters may be largely normal. In addition, unconjugated bilirubin in plasma is bound to albumin and transported to the hepatocytes, where it is subsequently separated from albumin and then enters the hepatocytes. A study has suggested that when hyperbilirubinemia exists for a long time (e.g., chronic biliary obstruction), bilirubin binds irreversibly to albumin; this kind of bilirubin is called δ-bilirubin (17).
Due to the long half-life of albumin, albumin-bound δ-bilirubin may remain in the plasma for a long time, and hyperbilirubinemia may continue for a long time even after the endoscopic or surgical removal of the biliary obstruction (18). The phenomenon may also be one of the reasons why Patient 2 continued to exhibit hyperbilirubinemia even after ERCP relieved the extrahepatic biliary obstruction. In addition, Patient 2 also had heterozygous mutations in other genes, such as UGT1A1, MAN2B1, and ACADSB. It is currently believed that only homozygous mutations in these genes cause disease; however, the question of whether heterozygous mutations affect the normal function of these proteins warrants further investigation. It may also be the reason why Patient 2 became critically ill so quickly.
Splenectomy is regarded as the most reliable and effective way to treat HS (19). The shape of RBCs cannot be changed after splenectomy; however, the destruction of RBCs can be greatly reduced, thereby substantially improving the symptoms of anemia and jaundice. In addition, long-term erythrocyte destruction may lead to the deposition of large amounts of iron in liver tissue, causing inflammation and fibrosis in the liver and even cirrhosis. Splenectomy may also effectively prevent the development of liver lesions in HS patients (20). Patient 1 in this article finally underwent splenectomy, and jaundice and anemia were significantly improved. Tamary et al. (21) reported that it was common for patients with HS combined with gallstones and relevant symptoms at the first visit. In a case report by Zhao et al. (10), splenectomy was performed in a patient with HS who had high total serum bilirubin of 443.7 µmol/L and whose ALT and AST levels were both significantly elevated; the patient’s bilirubin level decreased rapidly after surgery, and the patient’s liver function essentially returned to normal after surgery. Unfortunately, Patient 2 in this article refused surgical treatment despite high bilirubin levels.
Despite the similar clinical presentation of these 2 patients, the patients were treated differently due to the varying degrees of disease progression, which ultimately led to different outcomes. Thus, surgical treatment is a serious question for this group of HS patients who require splenectomy for rapid relief of hyperbilirubinemia and whose extremely high bilirubin levels may not tolerate the procedure.
In conclusion, by summarizing the diagnosis and treatment processes of these 2 patients, we have gained some experience. First, in the late stage of HS, the symptoms of anemia and hemolysis may not be obvious, and patients may present with splenomegaly, severe jaundice, and liver damage as the main manifestations and should be transferred to the hepatology department. Hepatologists should avoid misdiagnosis. Second, patients often present with severe hyperbilirubinemia but no primary disease in the liver, so liver-specific medications, such as hepatoprotection medicine, may be ineffective, and splenectomy should be performed as early as possible. ERCP should be performed promptly for patients with biliary obstruction, and a nasociliary drainage tube should be indwelled for decompression. After the obstruction is relieved, splenectomy and cholecystectomy should be actively considered. Third, while a liver biopsy is invasive, it helps a histopathological diagnosis to be made in patients with suspected intrahepatic cholestasis. Finally, timely genetic testing is necessary for the differential diagnosis of some patients with suspected inherited metabolic diseases.
Acknowledgments
We would like to thank all the patients and families involved in this article. We would also like to thank the Center of Infectious Diseases staff at West China Hospital of Sichuan University.
Funding: This work was supported by the Science and Technological Supports Project of Sichuan Province (No. 2020YFS0135) and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYGD20009).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5076/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5076/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from each patient or patient’s families for publication of these case reports and accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Da Costa L, Galimand J, Fenneteau O, et al. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev 2013;27:167-78. [Crossref] [PubMed]
- He BJ, Liao L, Deng ZF, et al. Molecular Genetic Mechanisms of Hereditary Spherocytosis: Current Perspectives. Acta Haematol 2018;139:60-6. [Crossref] [PubMed]
- Wu Y, Liao L, Lin F. The diagnostic protocol for hereditary spherocytosis-2021 update. J Clin Lab Anal 2021;35:e24034. [Crossref] [PubMed]
- Eber SW, Pekrun A, Neufeldt A, et al. Prevalence of increased osmotic fragility of erythrocytes in German blood donors: screening using a modified glycerol lysis test. Ann Hematol 1992;64:88-92. [Crossref] [PubMed]
- Wang C, Cui Y, Li Y, et al. A systematic review of hereditary spherocytosis reported in Chinese biomedical journals from 1978 to 2013 and estimation of the prevalence of the disease using a disease model. Intractable Rare Dis Res 2015;4:76-81. [Crossref] [PubMed]
- Farias MG. Advances in laboratory diagnosis of hereditary spherocytosis. Clin Chem Lab Med 2017;55:944-8. [Crossref] [PubMed]
- Agarwal AM, Nussenzveig RH, Reading NS, et al. Clinical utility of next-generation sequencing in the diagnosis of hereditary haemolytic anaemias. Br J Haematol 2016;174:806-14. [Crossref] [PubMed]
- Casale M, Perrotta S. Splenectomy for hereditary spherocytosis: complete, partial or not at all? Expert Rev Hematol 2011;4:627-35. [Crossref] [PubMed]
- Kalinke L, Rashid M. A cholestatic diagnostic dilemma. BMJ Case Rep 2013;2013:bcr2012008417. [Crossref] [PubMed]
- Zhao CY, Wang W, Liu YH, et al. Severe intrahepatic cholestasis and hemochromatosis secondary to hereditary spherocytosis. Zhonghua Gan Zang Bing Za Zhi 2010;18:552-3. [PubMed]
- Wree A, Canbay A, Müller-Beissenhirtz H, et al. Excessive bilirubin elevation in a patient with hereditary spherocytosis and intrahepatic cholestasis. Z Gastroenterol 2011;49:977-80. [Crossref] [PubMed]
- Zhou D, Chen YW, Cao HX, et al. Hyperbilirubinemia in a patient with hereditary spherocytosis and intrahepatic cholestasis. Journal of Practical Hepatology 2015;18:310-1.
- Zhang YD, Zuo NY, Zhang SS, et al. Hereditary spherocytosis with intrahepatic cholestasis caused by SPTB gene mutation in a case. Zhonghua Er Ke Za Zhi 2019;57:893-5. [PubMed]
- Boyer JL. Bile formation and secretion. Compr Physiol 2013;3:1035-78. [Crossref] [PubMed]
- Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol 2015;63:1023-37. [Crossref] [PubMed]
- Cooper RA, Jandl JH. The role of membrane lipids in the survival of red cells in hereditary spherocytosis. J Clin Invest 1969;48:736-44. [Crossref] [PubMed]
- Weiss JS, Gautam A, Lauff JJ, et al. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. N Engl J Med 1983;309:147-50. [Crossref] [PubMed]
- Bloomer JR, Berk PD, Howe RB, et al. Interpretation of plasma bilirubin levels based on studies with radioactive bilirubin. JAMA 1971;218:216-20. [Crossref] [PubMed]
- Schilling RF. Risks and benefits of splenectomy versus no splenectomy for hereditary spherocytosis--a personal view. Br J Haematol 2009;145:728-32. [Crossref] [PubMed]
- Videla LA, Valenzuela R. Perspectives in liver redox imbalance: Toxicological and pharmacological aspects underlying iron overloading, nonalcoholic fatty liver disease, and thyroid hormone action. Biofactors 2022;48:400-15. [Crossref] [PubMed]
- Tamary H, Aviner S, Freud E, et al. High incidence of early cholelithiasis detected by ultrasonography in children and young adults with hereditary spherocytosis. J Pediatr Hematol Oncol 2003;25:952-4. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


