
Classical ALK G1202R resistance mutation was identified in a lung adenocarcinoma patient with rare LOC388942-ALK fusion after sequential treatment with ALK-TKIs and anlotinib: a case report
Introduction
Anaplastic lymphoma kinase (ALK) gene rearrangements have been reported in approximately 5–7% of lung adenocarcinomas (LADCs) (1). ALK-positive LADC patients can significantly benefit from ALK tyrosine kinase inhibitors (ALK-TKIs). However, ALK fusion non-small cell lung cancer (NSCLC) is a heterogeneous disease. Distinct ALK fusions have different responses to various ALK-TKIs (first-generation: crizotinib; second-generation: alectinib, ceritinib, brigatinib, and ensartinib; third-generation: lorlatinib) (2-7). In addition, despite the impressive efficacy of ALK-TKIs in NSCLC patients, acquired resistance will inevitably develop (8,9). ALK inhibitors have two major resistance modes: ALK-dependent and non-ALK-dependent modes (10). The former mainly includes ALK kinase domain resistance mutations and increased copy numbers of ALK rearrangements. The latter includes bypass activation, such as emergence of MET and KRAS alterations, and histologic transformation, among others. It has been reported that different patterns of ALK fusion present with different stabilities of the resulting fusion proteins and different resistance mutation sites (11,12). Even among cancers harboring the same ALK fusion partner, such as EML4, unique fusion variants display a different biology—with clear differences in both duration of response and mechanism of resistance (13). Different ALK-TKI treatments also lead to different acquired resistance mutations due to their unique molecular structures and different binding patterns (14). For tumors that acquire an ALK-dependent resistance mechanism, treatment with another appropriate ALK-TKI that is tailored to that mutation may continue to provide antitumor therapeutic effects, while antitumor therapies other than ALK-TKIs would be preferred for tumors that acquire an ALK-independent resistance mechanism (15,16). Therefore, accurate molecular diagnosis of the ALK fusion pattern and dynamic monitoring of resistance mechanisms during treatment is key to achieving precision medicine and individualized treatment for optimal survival benefit to these patients. Here, we report that a lung cancer patient with a rare LOC388942-ALK fusion was sensitive to crizotinib but resistant to sequential ceritinib and alectinib and then developed a classical ALK G1202R resistance mutations after long-term anlotinib treatment, achieving 5-year overall survival (OS). This case highlights dynamic monitoring of gene alteration using next-generation sequencing (NGS) is necessary during the anti-tumor process. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5194/rc).
Case presentation
A 55-year-old Chinese male patient, with no history of smoking history and no family history of cancer, presented with left chest pain and dyspnea and was admitted to our hospital in August 2017. Physical examination showed his chest tender to palpation and decreased breath sounds in the left lung. Chest computed tomography (CT) found large pleural effusion and multiple round nodules in his left lung (Figure 1A). Pathological examination revealed the LADC staging as IVa (cT4N0M1a). Immunohistochemistry (IHC) showed ALK-V (+) (Ventana), which was also confirmed by fluorescence in situ hybridization (FISH). NGS results revealed a LOC388942-ALK fusion (L intergenic: A 20, 1.41%) (Figure 1B). He received first-line pemetrexed and cisplatin combined with antiangiogenic therapy for 6 cycles from August 2017 to April 2018, with a best response of partial response (PR). After the disease progressed, crizotinib was prescribed to the patient with a PR for 7 months from September 2018 to March 2019. When the primary lung lesion slowly progressed again, NGS was performed using blood samples that showed the same ALK fusion pattern as LOC388942-ALK fusion (L intergenic: A 20, 0.38%), without any other acquired molecular resistance events (Figure 1B). As the patient declined further chemotherapy and his renal function was inadequate for chemotherapy, ceritinib was administered from May 2019 to November 2019, during which the lesion still gradually enlarged. Meanwhile the renal function was deteriorated with high creatinine. Therefore, liquid biopsy performed again only revealed the same LOC388942-ALK fusion with a variant allele fraction of 1.11% and a TP53 mutation (exon 10 nonsense mutation, 0.61%). The patient started alectinib combined with radiotherapy (IMRT with a total dose of 60 Gy in 30 fractions was delivered to the tumor lesion in left lower lobe) in January 2020. The tumor responded to radiotherapy for the first 2 months but gradually enlarged again over the following 6 months, with the best response being stable disease (SD). After 8 months of alectinib treatment, the patient was unsuitable for chemotherapy due to his poor condition as well as his abnormal renal function. Anlotinib was utilized as a fifth-line treatment due to disease progression with no new NGS findings in October 2020. The tumor was responsive to antiangiogenic therapy. Thirteen months later, new lesions in the lower lobe of the right lung appeared and increased gradually. Meanwhile, retroperitoneal lymph nodes, including at hepatogastric ligament and para-aortic sites, were also enlarged, indicating definite progression of the tumor. Genetic testing by cfDNA NGS was performed again, which detected the LOC388942-ALK fusion (L intergenic: A 20, 0.50%) and the previously detected TP53 mutation (exon 10 nonsense mutation, 0.67%), but the classical ALK G1202R resistance mutation was also detected (0.22%), providing rationale for the patient to receive treatment with the third-generation ALK-TKI, namely, lorlatinib. However, the patient refused to receive lorlatinib targeting G1202R resistance mutations due to high fee and continued with anlotinib. He dead in August 2022, achieving 5-year OS. We summarized and described clonal evolution of the tumors during the whole clinical history (Figure 1C). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
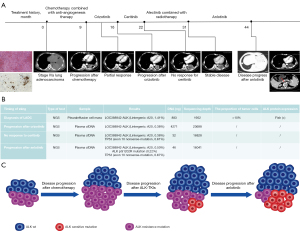
Discussion
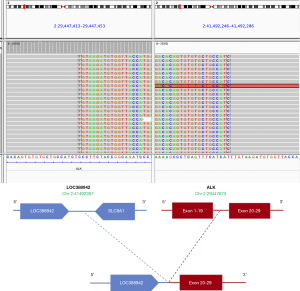
LOC388942-ALK fusions are very rare, accounting for approximately 0.01% (1/9,889) of NSCLC cases (17,18). To our knowledge, this study is the first to report the details of the clinical response and resistance mechanism of LOC388942-ALK (L intergenic: A20) fusion in ALK + NSCLC patients. LOC388942-ALK (L intergenic: A20) fusion occurred upon LOC388942 and SLC8A1 intergenic region rearrangement to the ALK kinase domain (exons 20–29) (Figure 2). In addition, in clinical practice, we also found another LOC388942-ALK fusion subtype: dual ALK fusions EML4-ALK (E6:A20) and LOC388942-ALK (L1:A20). For dual ALK fusions, the 5’ end of EML4 fused to the entire ALK kinase domain (exon 20–29), while LOC388942 (breakpoint in exon 1) rearranged to exons 1–19 of ALK (Figure S1). These two fusion mechanisms are different. As previous study has shown, two different breakpoint ALK variants may respond differently to ALK inhibitors, such as V1 and V3 of EML4-ALK (19). In this regard, the above two different LOC388942-ALK fusions may not have the same clinical response and resistance mechanisms. For the LOC388942-ALK (L intergenic: A20) fusion in our case, the patient achieved an optimal efficacy of PR with crizotinib as the second-line treatment after progression with first-line chemotherapy, demonstrating that this subtype of fusion site can be sensitive to crizotinib.
After the cancer developed crizotinib resistance, we performed continuous ctDNA monitoring during treatment, with no clear resistance mutation mechanism found over time. According to the literature, a direct switch to a second-generation ALK-TKI may be effective in a subset of patients after resistance to a first-generation ALK-TKI, even if no clear ALK secondary resistance site is found (15). Accordingly, we sequentially administered ceritinib and alectinib to the patient, while radiotherapy for the lung lesions was applied during treatment with alectinib. The pulmonary lesion enlarged slowly, suggesting that the tumor was at least to some extent insensitive to ceritinib and alectinib. After alectinib, as no specific resistance target emerged at that time, we chose anlotinib, a small-molecule multitarget TKI, to extend the patient’s progression-free survival (PFS) by another 13 months until comprehensive tumor progression occurred. Liquid biopsy was performed again, and this time, the classical resistance mutation ALK G1202R was identified, thus providing the patient with an opportunity to receive lorlatinib (20). However, the patient refused to receive lorlatinib targeting G1202R resistance mutations due to high fee and continued with anlotinib. He dead in August 2022, achieving 5-year OS. To our knowledge, this is the first report to describe detailed clinical information of the rare fusion site LOC388942-ALK. Based on the treatment experience of this patient, If he continue to take lorlatinib, longer OS might be observed. Dynamic detection of genetic changes for oncogene-addictive NSCLC can help patients achieve better survival rates. Further, according to current consensus, different generations of ALK-TKIs are more likely to produce different types of secondary resistance mutations, while different ALK fusion patterns may also affect their resistance mutation sites, as the expression efficiency and signaling strength of ALK fusion proteins are influenced by the fused partner genes. For example, two mutation patterns of R1192P and T1151M, which arise in NPM-ALK, have never been reported in ELM4-ALK fusion genes (12).
Another special feature of this case is that the patient did not develop a known resistance mutation during the course of continuous ALK-TKI therapy until after a year of subsequent relatively broad-spectrum anlotinib treatment. This may be due to the sensitivity of liquid biopsy in relation to tumor burden. But this phenomenon also highlights the continuous evolution and clonal evolution of tumors. As Stanková et al. (21) suggested the ALK fusion-prominent tumor population is gradually reduced to non-dominant ALK-positive clones under the continuous therapeutic pressure of ALK-TKIs, which is reflected in the slow resistance to ALK inhibitors, while other non-dominant clones that are not inhibited by ALK-TKIs gradually grow into the main body. After relatively broad-spectrum anticancer treatment with anlotinib, all tumor clones, including non-ALK-dependent tumor clones, could have been balanced, at which time the ALK G1202R tumor strain, which was latent in the non-dominant clone population but with higher malignancy, was reassessed, evolved toward the dominant clone again, and was finally detected.
This case suggests that in the process of antitumor therapy, real-time dynamic monitoring of tumor dominant subclones by ctDNA and NGS technologies and selecting the most appropriate treatment regimen according to the clonal population of the tumor are important strategies for the precise and individualized treatment of patients. In the era of precision targeted therapy, broad-spectrum antitumor treatment modalities, including chemotherapy and small-molecule multitarget drugs, still have value. How to combine the two therapies will naturally determine whether the longest survival and optimal treatment effect can be achieved for patients.
Conclusions
In conclusion, here we report for the first time that a LADC patient with a rare LOC388942-ALK fusion was sensitive to crizotinib but relatively resistant to ceritinib and alectinib. The patient acquired classical ALK G1202R resistance mutations after long-term anlotinib treatment. Distinct ALK fusions in NSCLC have different cancer biology, leading to different response to ALK-TKIs, even developed different resistance mechanism. Reporting the clinical details of rare ALK fusions in NSCLC is necessary to guide the treatment for clinicians and researchers.
Questions to be further discussed and considered
Question 1: What might be the reason that NGS of plasma cfDNA did not detect ALK resistance mutation? And what might be the mechanism of no response after the use of crizotinib and ceritinib?
Stephen V. Liu: This is speculative—could be due to limited sensitivity of the assay or a different mechanism of resistance.
Sang-Won Um: There is a possibility that the allele frequency of ALK resistance mutation might be below the detection limit of plasma NGS. In this case report, the patient was responsive to crizotinib and alectinib, but not ceritinib. Interestingly, Li et al. (22) also reported the benefit of sequential ALK-TKIs (alectinib and lorlatinib) for patients with two concurrent non-EML4-ALK rearrangements (LOC388942-ALK and LINC00211-ALK) (1). There was no information on the response of crizotinib and ceritinib for LOC388942-ALK rearrangement in the literature. The mechanism of no response after the use of ceritinib might be due to the intrinsic resistance of this rare non-EML4-ALK rearrangement (LOC388942-ALK) to ceritinib or the development non-ALK dependent bypass mechanisms.
Question 2: What might be the other mechanism underlying why the patient did not develop a known resistance site during the course of continuous ALK-TKI therapy until after a year of subsequent relatively broad-spectrum anlotinib treatment?
Stephen V. Liu: It’s hard to know for sure. One would not necessarily expect an ALK G1202R mutation to give the lung cancer a competitive advantage in the face of anlotinib. Why would anlotinib apply selective pressure to allow that clone to thrive? Perhaps anlotinib targeted other resistance clones, as you suggest, and 1,202 emerged in the absence of other, formerly robust clones, but hard to know.
Sang-Won Um: I think that the resistance mutation of ALK G1202R might not develop after anlotinib treatment. It might develop after the use of crizotinib or alectinib. There was no known literature about the development of ALK resistance mutation after anlotinib treatment in patients with ALK-rearranged NSCLC.
Question 3: How should this patient be treated after disease progression after lorlatinib treatment?
Stephen V. Liu: Docetaxel or a novel ALK TKI such as NVL-655 or TPX-0131.
Sang-Won Um: The patient already received pemetrexed and cisplatin combined with antiangiogenic therapy for 6 cycles as a first line treatment. One of possible treatment options after progression following lorlatinib treatment would be an IMpower 150 regimen (carboplatin/paclitaxel/bevacizumab/atezolizumab) which demonstrated an improvement in PFS in either EGFR mutant or ALK rearranged non-squamous NSCLC in post-hoc analysis (23). Since the main resistance mechanism of lorlatinib is the dual ALK mutations, double mutant active 4G ALK-TKIs (TPX-0131 or NVL-655) would be the future treatment options (24). They are now under phase 1/2 clinical trials (NCT04849273 and NCT05384626).
Acknowledgments
Thank you to Burning Rock Biotech (Guangzhou, China) for their support in the genetic analysis technology and Mengzhuang Bu for editing the figures. The authors also appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by the Wu Jieping Medical Foundation, China [grant No. 312150082].
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5194/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5194/coif). SWU serves as an unpaid editorial board member of Annals of Translational Medicine from September 2022 to August 2024. TQ is from Burning Rock Biotech. SVL reports that he is serving as consultant or advisory board member for Amgen, AstraZeneca, Bayer, Beigene, Blueprint, Boehringer-Ingelheim, Bristol-Myers Squibb, Catalyst, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Lilly, Merck/MSD, Novartis, Regeneron, Sanofi, Takeda, and Turning Point Therapeutics and receiving research grants (to institution) from Alkermes, Blueprint, Bristol-Myers Squibb, Elevation Oncology, Genentech, Gilead, Merck, Merus, Nuvalent, Pfizer, RAPT, Turning Point Therapeutics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Ou SI, Zhu VW, Nagasaka M. Catalog of 5’ Fusion Partners in ALK-positive NSCLC Circa 2020. JTO Clin Res Rep 2020;1:100015. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Isozaki H, Takigawa N, Kiura K. Mechanisms of Acquired Resistance to ALK Inhibitors and the Rationale for Treating ALK-positive Lung Cancer. Cancers (Basel) 2015;7:763-83. [Crossref] [PubMed]
- Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol 2022;19:499-514. [Crossref] [PubMed]
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res 2012;18:4682-90. [Crossref] [PubMed]
- Amin AD, Li L, Rajan SS, et al. TKI sensitivity patterns of novel kinase-domain mutations suggest therapeutic opportunities for patients with resistant ALK+ tumors. Oncotarget 2016;7:23715-29. [Crossref] [PubMed]
- Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol 2018;36:1199-206. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Park S, Han J, Sun JM. Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 2019;127:66-8. [Crossref] [PubMed]
- Zhang Y, Zeng L, Zhou C, et al. Detection of Nonreciprocal/Reciprocal ALK Translocation as Poor Predictive Marker in Patients With First-Line Crizotinib-Treated ALK-Rearranged NSCLC. J Thorac Oncol 2020;15:1027-36. [Crossref] [PubMed]
- Zhao R, Zhang J, Han Y, et al. Clinicopathological Features of ALK Expression in 9889 Cases of Non-small-Cell Lung Cancer and Genomic Rearrangements Identified by Capture-Based Next-Generation Sequencing: A Chinese Retrospective Analysis. Mol Diagn Ther 2019;23:395-405. [Crossref] [PubMed]
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer 2018;142:2589-98. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Stanková K, Brown JS, Dalton WS, et al. Optimizing Cancer Treatment Using Game Theory: A Review. JAMA Oncol 2019;5:96-103. [Crossref] [PubMed]
- Li Z, Li P, Yan B, et al. Sequential ALK inhibitor treatment benefits patient with leptomeningeal metastasis harboring non-EML4-ALK rearrangements detected from cerebrospinal fluid: A case report. Thorac Cancer 2020;11:176-80. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Ou SI, Nagasaka M, Brazel D, et al. Will the clinical development of 4th-generation "double mutant active" ALK TKIs (TPX-0131 and NVL-655) change the future treatment paradigm of ALK+ NSCLC? Transl Oncol 2021;14:101191. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


