
Prognostic factors of hearing outcome in patients with chronic suppurative otitis media following tympanoplasty: a retrospective cohort study
Introduction
Chronic suppurative otitis media (CSOM) is a common chronic infectious disease of the middle ear cleft characterized by persistent perforation of the tympanic membrane with recurrent or persistent discharge from the middle ear (1). CSOM frequently causes hearing impairment (2) and even more serious intracranial [e.g., brain abscess and meningitis (3)] and extracranial [e.g., subperiosteal abscess, labyrinthitis, and facial nerve palsy (4)] complications. CSOM is one of the main causes of hearing disabilities, which seriously affect the physical and mental health as well as the quality of life of patients (5).
At present, the primary treatment for CSOM is surgical management, mainly including tympanoplasty and mastoidectomy (6). The goal of tympanoplasty is to remove the lesion and restore the function of the middle ear (7,8). Numerous factors have been studied to determine their impact on postoperative hearing improvement. Understanding these prognostic factors is crucial for the development of a personalized surgical plan. In 1992, Black proposed that surgical, prosthetic, infection, tissue, and eustachian (SPITE) are five prognostic factors of the surgical outcomes of ossiculoplasty (9). Some studies have demonstrated that the surgical outcome depends on several factors, including preoperative tympanic membrane status, size and location of the perforation, duration of the dry period of the diseased ear, normal opposite ear, the status of malleus handle, type of surgical technique, smoking, and surgeon experience (10-16). However, research on the factors affecting the functional outcome of tympanoplasty in CMOS is limited. In the above studies, the conclusions may be inconsistent due to different evaluation criteria, incomplete research factors, and small sample size. The postoperative results are affected by many factors, such as the functional status of the eustachian tube, the choice of surgical methods, the nature of the prosthesis, the skills of the operator, and the differences between different individuals. There is a lack of large sample and multiple factors to conduct statistical analysis and research on the influence of demography, audiology, clinical and disease factors on the prognosis of CSOM.
Albu et al. demonstrated that the status of the mucosal lining, availability of the malleus handle, tympanic membrane perforation, and mastoidectomy are prognostic factors of the functional outcome of tympanoplasty (17). van der Veen et al. reported that the most important prognostic factor of tympanoplasty in CMOS is a history of recurrent episodes of acute otitis media (18). Due to the differences in study design and grouping, the definition of success, surgical methods, and the experience levels of the surgeons, it is difficult to directly compare the inconsistent findings of these studies. On the other hand, these results also indicate that the prognostic factors of surgical management in CMOS are complicated and require further investigation. The purpose of this study was to investigate the prognostic factors of hearing outcome in CSOM patients following tympanoplasty. The main aim of this study was to compare this outcome measure with a range of variables that might be expected to affect the hearing outcome using multivariate logistic regression analysis. This method of analysis was chosen because it was considered likely that confounding variables would produce misleading results if univariate analysis was employed. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4800/rc).
Methods
Study subjects
A total of 179 CSOM patients receiving surgery in the Otorhinolaryngology Head and Neck Surgery of Nanfang Hospital of Southern Medical University between January 2016 and December 2019 were retrospectively included in this study. The inclusion criteria were as follows: (I) the ear discharge, pure tone hearing threshold test, otoscope examination, and temporal bone computed tomography (CT) results met the diagnostic criteria of CSOM based on the clinical classification of otitis media and surgical classification guidelines [2012] (19); (II) patients who received surgical treatment by the same surgeon in the otology treatment group; (III) preoperative hearing impairment was conductive or mixed hearing impairment; and (IV) the surgical method was pure intact canal wall mastoidectomy, intact canal wall mastoidectomy plus tympanoplasty, or canal wall down mastoidectomy plus tympanoplasty. Patients were excluded based on the following criteria: (I) patients who had undergone previous middle ear surgery and those with incomplete data; (II) patients with acute otitis externa; and (III) those with a history of ear trauma and traumatic tympanic perforation.
All patients underwent comprehensive examinations, such as the pure-tone hearing threshold test and otoscope examination 1 week before surgery and 4 weeks postoperatively. The patients were divided into an effective group [air-bone gap (ABG) ≤20 dB, n=132] and a non-effective group (ABG >20 dB, n=47) according to their postoperative ABG value (20). All patients who participated in this study signed an informed consent form, and this study was approved by the ethical institute of Nanfang Hospital, Southern Medical University (No. NFEC-2021-248). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Surgical management
All cases were completed by the same surgeon, and all patients were operated on under general anesthesia. The surgical method was determined according to the extent of the lesion (19). Among the included cases, 120 underwent simple tympanoplasty, 55 underwent intact canal wall mastoidectomy plus tympanoplasty, and four received completed canal wall down mastoidectomy plus tympanoplasty.
Data collection and definition
The following clinical factors were recorded: gender, age, disease duration, type of preoperative hearing impairment, preoperative ear discharge, middle ear cholesteatoma, tympanic perforation size, infection status (intraoperative detection of purulent secretions in the tympanic cavity, divided into the quiescent period and active period), Eustachian tube function, tympanic sclerosis, ossicular chain status, tympanic membrane repair material (temporal muscle fascia, mastoid periosteum), and surgical methods.
The ossicular chain state was divided into three categories: (I) the ossicular chain was complete, and the ossicular chain was free to move without fixation; (II) the ossicular chain was complete, but part or all of the ossicles were fixed; (III) the ossicular chain was incomplete, with an absence of ossicles. Tympanic perforation was classified according to the size of the ear endoscopic photos (21) as follows: small perforation (diameter <3 mm); medium perforation (diameter 3–5 mm); and large perforation (diameter >5 mm).
Efficacy evaluation
All patients underwent pure-tone hearing threshold detection 1 week before surgery and 4 weeks after surgery. The ABG and speech reception threshold (SRT) were compared pre- and post-operatively at four frequencies (500 Hz, 1 kHz, 2 kHz, and 4 kHz). A postoperative mean ABG ≤20 dB was considered to indicate the effectiveness of hearing improvement.
Postoperative follow-up
Four weeks after the operation, the patients were followed up for re-examination of the ear endoscopy and pure tone hearing threshold test. The recovery of tympanic membrane of patients was observed through otoendoscope, whether there was ear effusion, perforation of tympanic membrane, etc. The pure tone hearing results were mainly compared with the results of 1 week before operation. All patients were followed up regularly thereafter.
Statistical analysis
Normally distributed measurement data were expressed as the mean ± standard deviation (SD). Univariate and multivariate binary logistic regression analysis was conducted to analyze the factors affecting the postoperative hearing prognosis in CSOM. The analysis of related factors affecting the efficacy and prognosis took whether the treatment was effective as the research factors of this study. The gender, age, disease duration (years), type of preoperative hearing impairment, preoperative ear discharge, eustachian tube function, tympanic, tympanic perforation site, ossicular chain status, infection status and tympanic sclerosis were included in the single factor analysis, and then the factors with statistical differences were analyzed by multivariate logistic regression analysis. P<0.05 indicated that the difference was statistically significant. All of the data were statistically analyzed using SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA).
Results
Patient demographic and clinical characteristics
A total of 179 CSOM patients (179 ears) were retrospectively analyzed, including 69 males (38.5%) and 110 females (61.5%), with a mean age of 38.44±12.73 (range, 17–73) years. There were 88 cases (49.2%) in the left ear and 91 cases (50.8%) in the right ear. The mean disease duration was 16.38±14.24 years (range, 3 months–50 years).
The 179 CSOM patients were divided into an effective group (n=132, 73.7%) and a non-effective group (n=47, 26.3%) according to their postoperative efficacy. The patients’ demographic and clinical characteristics were compared in Table 1. There were no significant differences in gender, age, and disease duration between the two groups (all P<0.01; Table 1). However, the preoperative hearing type (P=0.048), middle ear cholesteatoma (P=0.017), ossicular chain status (P<0.001), tympanic perforation size (P<0.001), tympanic perforation site (P<0.001), infection status (P<0.037), and tympanum sclerosis (P=0.001) differed markedly between the effective and non-effective groups (Table 1).
Table 1
| Study group | Effective | Non-effective | P |
|---|---|---|---|
| Case (%) | 132 (73.7) | 47 (26.3) | – |
| Gender, n (%) | 0.383 | ||
| Male | 48 (36.4) | 21 (44.7) | |
| Female | 84 (63.6) | 26 (55.3) | |
| Age (years), mean ± SD | 38.44±12.794 | 38.45±12.68 | 0.997 |
| Disease duration (years), mean ± SD | 15.43±13.53 | 19.01±15.91 | 0.141 |
| Type of preoperative hearing impairment, n (%) | 0.048* | ||
| Conductive | 93 (70.5) | 25 (53.2) | |
| Mixed deafness | 39 (29.5) | 22 (46.8) | |
| Preoperative ear discharge, n (%) | 1.00 | ||
| No | 19 (14.4) | 7 (14.9) | |
| Yes | 113 (85.6) | 40 (85.1) | |
| Middle ear cholesteatoma, n (%) | 0.017* | ||
| No | 124 (93.9) | 38 (80.9) | |
| Yes | 8 (6.1) | 9 (19.1) | |
| Ossicular chain status, n (%) | <0.001* | ||
| Complete | 17 (12.9) | 13 (27.7) | |
| Fixed | 100 (75.8) | 16 (34.0) | |
| Interrupted | 15 (11.4) | 18 (38.3) | |
| Tympanic perforation size, n (%) | <0.001* | ||
| <3 mm | 0 (0.0) | 2 (4.3) | |
| 3–5 mm | 15 (11.4) | 8 (17.0) | |
| >5 mm | 117 (88.6) | 37 (78.7) | |
| Tympanic perforation site, n (%) | <0.001* | ||
| Tension part | 124 (93.9) | 33 (70.2) | |
| Relaxation part | 4 (3.0) | 10 (21.3) | |
| Tension and relaxation parts | 4 (3.0) | 4 (8.5) | |
| Repair material, n (%) | 0.115 | ||
| Temporal muscle fascia | 101 (76.5) | 18 (38.3) | |
| Mastoid periosteum | 31 (23.5) | 29 (61.7) | |
| Infection status, n (%) | 0.037* | ||
| Active period | 88 (66.7) | 25 (53.2) | |
| Quiescent period | 44 (33.3) | 22 (46.8) | |
| Tympanic sclerosis, n (%) | <0.001* | ||
| No | 44 (33.3) | 24 (51.1) | |
| Yes | 88 (66.6) | 23 (48.9) | |
| Surgical approach, n (%) | 0.158 | ||
| Tympanoplasty | 101 (76.5) | 19 (40.4) | |
| Complete mastoidectomy + tympanoplasty | 28 (21.2) | 27 (57.4) | |
| Open mastoidectomy + tympanoplasty | 3 (2.3) | 1 (2.1) | |
| Eustachian tube function, n (%) | 0.383 | ||
| Bad | 43 (32.6) | 21 (44.7) | |
| Good | 89 (67.4) | 26 (55.3) |
*P<0.05, comparison between the two groups. SD, standard deviation.
Comparison of the pre- and post-operative ABG/SRT
The pre- and post-operative ABG/SRT in both groups were compared. In the effective group, the SRT (36.88±15.54 vs. 27.68±13.75, P<0.001) and ABG (18.53±9.30 vs. 10.89±5.07, P<0.001) values were significantly reduced after surgery (Figure 1). However, in the non-effective group, both the SRT (51.54±14.91 vs. 49.52±15.24, P=0.07) and ABG (27.05±7.19 vs. 26.60±5.22, P=0.67) values were not notably dissimilar pre- and post-operatively (Figure 1).
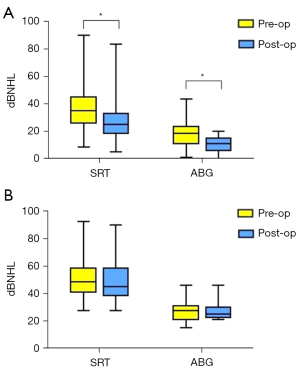
Comparison of the postoperative increases in ABG and SRT between the two groups
As shown in Figure 2, the postoperative SRT decrease was significantly higher in the effective group than in the non-effective group (9.20±9.69 vs. 2.021±7.34, P<0.001). Moreover, the postoperative ABG decrease was considerably higher in the effective group than in the non-effective group (7.64±8.57 vs. 0.45±7.322, P<0.001).
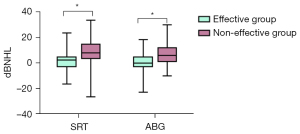
Multivariate logistic regression analysis of the prognostic factors of CSOM
Multivariate logistic regression analysis was performed to investigate prognostic factors of CSOM. As shown in Table 2, the type of preoperative hearing impairment [odds ratio (OR) =2.378; 95% confidence interval (CI): 1.084 to 5.209; P=0.031] and ossicular chain status were the prognostic factors associated with postoperative hearing improvement in CSOM patients. Compared to patients with an interrupted ossicular chain preoperative, those with a complete ossicular chain (OR =1.080; 95% CI: 0.364 to 0.325) and fixed ossicular chain (OR =4.430; 95% CI: 1.691 to 11.606) had better postoperative hearing improvement.
Table 2
| Groups | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Case (%) | 132 (73.7) | 47 (26.3) | |||
| Gender | 0.707 (0.360–1.390) | 0.315 | |||
| Male | |||||
| Female | |||||
| Age (years) | 1.00 (0.974–1.027) | 0.997 | |||
| Disease duration (years) | 0.983 (0.961–1.006) | 0.142 | |||
| Type of preoperative hearing impairment | 2.098 (1.059–4.159) | 0.034 | |||
| Conductive | 2.378 (1.084–5.209) | 0.031* | |||
| Mixed deafness | 1 | ||||
| Preoperative ear discharge | 0.961 (0.376–2.456) | 0.933 | |||
| No | |||||
| Yes | |||||
| Middle ear cholesteatoma | 3.671 (1.325–10.174) | 0.012 | |||
| No | |||||
| Yes | |||||
| Ossicular chain status | |||||
| Complete | 1.569 (0.580–4.246) | 0.375 | 1.080 (0.364–0.325) | 0.890 | |
| Fixed | 7.500 (3.158–17.810) | 0.000 | 4.430 (1.691–11.606) | 0.002* | |
| Interrupted | 1 | 1 | |||
| Tympanic perforation size | |||||
| <3 mm | 0.000 (0.000–0.000) | 0.999 | |||
| 3–5 mm | 0.593 (0.233–1.509) | 0.273 | |||
| >5 mm | 1 | ||||
| Repair material | 5.249 (2.574–10.705) | 0.000 | |||
| Temporal muscle fascia | |||||
| Mastoid periosteum | |||||
| Infection status | 1.760 (0.894–3.466) | 0.102 | |||
| Active period | |||||
| Quiescent period | |||||
| Tympanic sclerosis | 0.479 (0.244–0.943) | 0.033 | |||
| No | |||||
| Yes | |||||
| Surgical approach | |||||
| Tympanoplasty | 1.772 (0.175–17.952) | 0.628 | |||
| Complete mastoidectomy + tympanoplasty | 0.346 (0.034–3.532) | 0.370 | |||
| Open mastoidectomy + tympanoplasty | 1 | ||||
| Eustachian tube function | 0.598 (0.303–1.181) | 0.139 | |||
| Bad | |||||
| Good | |||||
*P<0.05, comparison between the two groups. CSOM, chronic suppurative otitis media; OR, odds ratio; CI, confidence interval.
Discussion
The integrity of the tympanic membrane and the ossicular chain plays a key role in the sound transmission of the middle ear (22). The severity of hearing impairment is closely related to each component in the middle ear sound transmission system, such as the location and size of the tympanic perforation, the degree of damage to the ossicular chain, the extent of lesion invasion, and the nature of the middle ear lesion. In this study, we investigated the prognostic factors of hearing outcome in CSOM patients following tympanoplasty. Multivariate logistic regression analysis showed that type of preoperative hearing impairment and preoperative ossicular chain status were the only two prognostic factors associated with postoperative hearing improvement in CSOM patients.
The impact of CSOM on hearing is mainly manifested as mild and moderate conductive hearing loss, as well as severe mixed hearing loss. The main causes of hearing loss in the early stage of CSOM are tympanic perforation, ossicular chain lesions, and tympanic sclerosis, which affect the mobility of the oval and round windows, causing conductive hearing loss (23). Clinically, some CSOM patients have conductive hearing loss combined with sensorineural hearing loss. It has been shown that inflammation of the middle ear in CSOM can increase the permeability of the round window, allowing bacteria and toxic substances in the pus of the middle ear to enter the inner ear. Consequently, the inner ear hair cells and the cochlear nerve are damaged, impairing sound perception or nerve impulse conduction, eventually leading to irreversible sensorineural hearing loss (24). In general, CSOM patients with severe disease and long disease duration have significant damage to the inner ear hair cells. Our results showed that the postoperative hearing improvement was 2.378 times better in patients with preoperative conductive deafness than in those with preoperative mixed deafness. Patients with mixed deafness generally have severe middle ear disease and longer disease duration, which is accompanied by inner ear damage and irreversible sensorineural hearing loss; as a result, these patients have worse postoperative hearing improvement.
The ossicular chain connects the tympanic membrane and the vestibular window. It plays a role in the conduction and amplification of sound, and its integrity is closely related to hearing. In the case of tympanic perforation and ossicular chain interruption, the amplification gain function is affected due to increased quality and stiffness, which in turn affects the sound transmission function, eventually resulting in conductive hearing impairment. Following tympanic perforation in CSOM patients, the three ossicles in the ossicular chain (the incus, stapes, and malleus) are sequentially damaged (25). As the disease progresses in CSOM patients, ossicular chain abnormalities generally manifest as completed but fixed, followed by an interrupted ossicular chain.
A previous study showed that a fixed ossicular chain has less effect on hearing loss than an interrupted ossicular chain (26). Moreover, the destruction and interruption of the ossicular chain usually occur in the presence of middle ear cholesteatoma or middle ear granulation (26). After surgical removal of the sclerotic lesions around the ossicular chain, the mobility of the fixed ossicular chain can be completely or partially restored. According to the histopathological classification of tympanosclerotic plaques by Selcuk et al. (27), the fixed ossicular chain in this study mainly belonged to type II tympanosclerosis (i.e., the malleus and incus are covered by sclerosis foci, the ossicular chain is fixed, but the stapes is intact and moves well) and type III tympanosclerosis (i.e., the sclerotic lesion involves the stapes so that the stapes is completely fixed). In this study, the ossicular chain fixation was mainly type II and type III, which not only exhibited conductive hearing loss but also mixed hearing loss. The mechanism at play here may be attributable to long-term inflammatory factor stimulation, immune injury, toxins, long-term use of inner ear edema ototoxicity drugs, as well as cell degeneration and even necrosis.
Other factors affecting the bone conduction pathway, such as the Acoustic Energy Radiation of the external auditory meatus, stiffness of the ossicular chain, pathological and structural changes of the middle ear, and limitation of the activity of the two windows, can induce changes to the bone conduction hearing threshold. However, it is also possible that the calcification focus, granulation, and scarring of the tympanosclerosis block vestibular window and cochlear window can cause false bone conduction decline, which can be recovered with the removal of the calcification focus and the reduction of mass inertia. Our analysis found that patients with ossicular chain fixation had 4.430 times better postoperative hearing outcomes than those with an interrupted ossicular chain. One possible explanation is that following surgical removal of the surrounding calcification, granulation, and scars, the ossicular chain can partially or fully restore its mobility, thereby resulting in a significant reduction in postoperative ABG. Our findings are consistent with the observation that the air conduction threshold and the difference between the air and bone conduction thresholds are both considerably greater in patients with an interrupted ossicular chain than those with a complete ossicular chain (28).
Moreover, it has been reported that the mean bone conduction value and 1, 2, 4 kHz bone conduction threshold of patients with an intact and well-moving ossicular chain differ markedly from those with ossicular chain fixation and ossicular chain interruption (29). In a pre-operative hearing analysis of 148 patients (176 ears) with CSOM, Zhu et al. (26) reported an air-conduction speech frequency threshold of <40 decibel normal hearing level (dBNHL) and air-bone conduction difference of <30 dBNHL in patients with normal ossicular chain. Patients with ossicular chain disruption had a higher air-bone conduction difference than those with intact ossicular chain, and the average air-bone conduction frequency threshold was >55 dBNHL and the air-bone conduction difference was >40 dBNHL in patients with a broken ossicular chain. Also, the air-bone conduction difference was larger than that in patients with an intact ossicular chain, the air-bone conduction difference was larger in patients with a broken ossicular chain compared to those with an intact ossicular chain, and the ossicular chain disruption usually occurred in the presence of middle ear cholesteatoma or granulation of the middle ear (26). The difference in the air conduction hearing threshold and air-bone conduction in ears with a broken ossicular chain was markedly greater than that in ears with an intact ossicular chain (28). The degree of damage to the ossicular chain was notably different between tympanosclerosis and cholesteatoma, and ossicular chains with tympanosclerosis were mainly characterized by insect-like bone destruction. The degree of this destruction was mild, which may have been induced by blood supply disturbance resulting from the ossicular lesion encasing the ossicular bone, but not directly caused by the ossicular lesion.
Therefore, we believe that the type of ossicular chain fixation observed in this study is 4.430 times more effective than the type of ossicular chain disruption, which may be because ossicular chain fixation patients have milder lesions, and thus, their hearing loss is mainly due to granulation, scarring, calcification, and other tissues involved in the ossicular chain. It may also be related to the ossicular chain in general after careful removal of the surrounding lesions, which restores activity to a part or all of the ossicular chain, leading to restoration of the middle ear sound transmission structure. This results in a more significant decrease in the ABG after surgery.
Understanding the preoperative hearing condition and ossicular chain status of patients with CSOM, adopting corresponding treatment methods according to their different ossicular chain statuses, and fully comprehending the related factors will help to evaluate the prognosis of these patients. However, there are some limitations in this study that should be noted. Firstly, the current study was limited by its retrospective nature and relatively small sample size. In addition, the follow-up time was short. In the future, a large, well-designed prospective trial should be conducted to validate the findings of this study.
Conclusions
In summary, our study showed that the type of preoperative hearing impairment and the status of the ossicular chain are prognostic factors of the hearing outcome in CSOM patients following tympanoplasty. These findings may help to predict the postoperative prognosis of patients and facilitate the development of corresponding treatment strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4800/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4800/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4800/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All patients who participated in this study signed an informed consent form, and this study was approved by the ethical institute of Nanfang Hospital, Southern Medical University (No. NFEC-2021-248). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tang Y, Lian B, Zhang M, et al. Sensorineural damage in chronic suppurative otitis media with and without cholesteatoma: a comparative study. Ann Transl Med 2022;10:778. [Crossref] [PubMed]
- Aarhus L, Tambs K, Kvestad E, et al. Childhood Otitis Media: A Cohort Study With 30-Year Follow-Up of Hearing (The HUNT Study). Ear Hear 2015;36:302-8. [Crossref] [PubMed]
- Parmar BD, Jha S, Sinha V, et al. A study of complications of chronic suppurative otitis media at tertiary care hospital. Int J Otorhinolaryngol Head Neck Surg 2020;6:330. [Crossref]
- Sharma N, Jaiswal AA, Banerjee PK, et al. Complications of Chronic Suppurative Otitis Media and Their Management: A Single Institution 12 Years Experience. Indian J Otolaryngol Head Neck Surg 2015;67:353-60. [Crossref] [PubMed]
- Baumann I, Gerendas B, Plinkert PK, et al. General and disease-specific quality of life in patients with chronic suppurative otitis media—a prospective study. Health Qual Life Outcomes 2011;9:48. [Crossref] [PubMed]
- Horvath T, Lukacs D, Horvath B, et al. Does The Type of Ossicular Chain Lesion Affect Outcomes in Chronic Suppurative Otitis Media Without Cholesteatoma? J Int Adv Otol 2019;15:28-33. [Crossref] [PubMed]
- Verhoeff M, van der Veen EL, Rovers MM, et al. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol 2006;70:1-12. [Crossref] [PubMed]
- Webb BD, Chang CY. Efficacy of tympanoplasty without mastoidectomy for chronic suppurative otitis media. Arch Otolaryngol Head Neck Surg 2008;134:1155-8. [Crossref] [PubMed]
- Black B. Ossiculoplasty prognosis: the spite method of assessment. Am J Otol 1992;13:544-51. [PubMed]
- Bared A, Angeli SI. Malleus handle: determinant of success in ossiculoplasty. Am J Otolaryngol 2010;31:235-40. [Crossref] [PubMed]
- Onal K, Uguz MZ, Kazikdas KC, et al. A multivariate analysis of ntological, surgical and patient-related factors in determining success in myringoplasty. Clin Otolaryngol 2005;30:115-20. [Crossref] [PubMed]
- Emir H, Ceylan K, Kizilkaya Z, et al. Success is a matter of experience: type 1 tympanoplasty: influencing factors on type 1 tympanoplasty. Eur Arch Otorhinolaryngol 2007;264:595-9. [Crossref] [PubMed]
- Pfammatter A, Novoa E, Linder T. Can myringoplasty close the air-bone gap? Otol Neurotol 2013;34:705-10. [Crossref] [PubMed]
- Lee P, Kelly G, Mills RP. Myringoplasty: does the size of the perforation matter? Clin Otolaryngol Allied Sci 2002;27:331-4. [Crossref] [PubMed]
- Becvarovski Z, Kartush JM. Smoking and tympanoplasty: implications for prognosis and the Middle Ear Risk Index (MERI). Laryngoscope 2001;111:1806-11. [Crossref] [PubMed]
- De Vos C, Gersdorff M, Gérard JM. Prognostic factors in ossiculoplasty. Otol Neurotol 2007;28:61-7. [Crossref] [PubMed]
- Albu S, Babighian G, Trabalzini F. Prognostic factors in tympanoplasty. Am J Otol 1998;19:136-40. [PubMed]
- van der Veen EL, Schilder AG, van Heerbeek N, et al. Predictors of chronic suppurative otitis media in children. Arch Otolaryngol Head Neck Surg 2006;132:1115-8. [Crossref] [PubMed]
- Chinese Medical Association Otolaryngology Head and Neck Surgery Branch Otology Group. Guidelines for clinical classification and surgical classification of otitis media (2012). Chinese Journal of Otorhinolaryngology Head and Neck Surgery 2013;48:5.
- Roth JA, Pandit SR, Soma M, et al. Ossicular chain reconstruction with a titanium prosthesis. J Laryngol Otol 2009;123:1082-6. [Crossref] [PubMed]
- Acuin J. Chronic suppurative otitis media. BMJ Clin Evid 2007;2007:0507.
- Luers JC, Hüttenbrink KB. Surgical anatomy and pathology of the middle ear. J Anat 2016;228:338-53. [Crossref] [PubMed]
- Redaelli de Zinis LO, Campovecchi C, Parrinello G, et al. Predisposing factors for inner ear hearing loss association with chronic otitis media. Int J Audiol 2005;44:593-8. [Crossref] [PubMed]
- Amali A, Hosseinzadeh N, Samadi S, et al. Sensorineural hearing loss in patients with chronic suppurative otitis media: Is there a significant correlation? Electron Physician 2017;9:3823-7. [Crossref] [PubMed]
- Bayat A, Saki N, Nikakhlagh S, et al. Ossicular chain defects in adults with chronic otitis media. Int Tinnitus J 2019;23:6-9. [Crossref] [PubMed]
- Zhu F, Sun M, Hua H. Destruction of ossicular chain and its impact on hearing of patients with chronic suppurative otitis media. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008;22:312-4. [PubMed]
- Selcuk A, Ensari S, Sargin AK, et al. Histopathological classification of tympanosclerotic plaques. Eur Arch Otorhinolaryngol 2008;265:409-13. [Crossref] [PubMed]
- Javia LR, Ruckenstein MJ. Ossiculoplasty. Otolaryngol Clin North Am 2006;39:1177-89. [Crossref] [PubMed]
- Chen J, Zheng Y. Destrution of ossicular chain and it’s impact on hearing of patients with tympanosclerosis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013;27:27-30. [PubMed]
(English Language Editor: A. Kassem)


