
The effects of external diaphragmatic pacing on diaphragm function and weaning outcomes of critically ill patients with mechanical ventilation: a prospective randomized study
Introduction
Mechanical ventilation (MV) is an important treatment for the rescue of critically ill patients, but the difficulty of weaning may lead to many complications, resulting in much strain on medical resources in the society and on individual families (1). Stieff et al. has reported that about 12–38% of mechanically ventilated patients who successfully pass a spontaneous breathing trial (SBT) may still experience weaning failure (2). Recently, it has been recognized that ventilator-induced diaphragmatic dysfunction (VIDD) is a critical influencing factor for weaning difficulty (3,4). Levine et al. demonstrated that 18 to 69 hours of complete ventilator-controlled ventilation resulted in an approximately 50% decline in the cross-sectional area of the diaphragm myofibers (5). Demoule et al. has also reported that diaphragmatic dysfunction occurs commonly in critically ill patients, with the incidence as high as 64% when patients are transferred to the intensive care unit (ICU) (6). Other reports have suggested that patients on MV can not only retain spontaneous breathing to reduce ventilation time, but also demonstrate improved diaphragm muscle activity, which can alleviate the atrophy of the diaphragm muscle due to disuse and alleviate the progression of diaphragm dysfunction (7-9). Therefore, monitoring and evaluation of VIDD is critical. Numerous studies have found that diaphragm excursion (DE) and diaphragm thickening fraction (DTF) can effectively reflect diaphragm function which are closely related to success rate of weaning (7-10). Moreover, measurement of DE and DTF using ultrasound to assess diaphragm structure (thickness, thickening) and mobility (caudal displacement) appears to be feasible and reproducible (10).
A recent investigation discovered that passive movement of skeletal muscles in ICU patients through neuromuscular electrical stimulation can significantly increase the strength of muscle contractions (11). Diaphragm neuromuscular electrical stimulation has become a research hotspot in recent years as the diaphragm is the most important respiratory muscle of the human body. External diaphragmatic pacing (EDP) is a passive respiratory muscle exercise methodology which stimulates the phrenic nerve to stimulate the diaphragm to passively contract regularly and effectively, and to promote the recovery of diaphragmatic dysfunction. It has been shown to effectively improve diaphragmatic dysfunction and reduce duration of MV (DMV) for patients with spinal cord injuries (SCIs) (11) and amyotrophic lateral sclerosis (ALS) (12,13). While EDP has been approved by the Food and Drug Administration (FDA) for clinical use, there is a paucity of data examining the effects of EDP on mechanically ventilated patients, especially in relation to DE and the success rate of weaning.
Therefore, this current study explored the effects of EDP on the diaphragm in mechanically ventilated patients, as well as its effects on the success rate of weaning, the DMV, and the ICU length of stay (ILOS). We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4145/rc).
Methods
Participants and study design
A prospective, double-blind, randomized, controlled trial was designed to study the effects of EDP therapy on patients with MV. Patients who underwent MV in the ICU of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University from September 2019 to December 2020 were enrolled and randomly divided into an experimental group (n=27) and a control group (n=24) using a random number generator (Figure S1). Participants were blinded to the treatment they received, while a tester who was blinded to the treatment and grouping of the patients recorded the DE, DTF, DMV, ILOS, and the average survival time in the two groups. The mean follow-up time was 1 year, and the follow-up was terminated in September 2021. The following inclusion criteria were applied: (I) age ≥18 years; and (II) expected MV time ≥3 days. The following exclusion criteria were applied: (I) pregnant women; (II) patients with hemodynamic instability and/or shock; (III) patients with a history of cervical SCI or neuromuscular disease; (IV) patients presenting with a history of phrenic nerve palsy; (V) patients with a body mass index (BMI) ≥40 kg/m2; (VI) patients who presented with contraindications to percutaneous diaphragm pacing (such as pneumothorax and cardiac pacemaker implantation); and (VII) patients who refused to sign the informed consent form for this study. Signed informed consent forms were obtained from all enrolled patients and their families. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. 2019-23).
Materials and methods
Patients in the control group (n=24) received routine MV as follows: the control pressure support level was adjusted in the pressure controlled (PC) or ventilation controlled (VC) mode to achieve a tidal volume of 8–10 mL/kg, saturation of pulse oxygen (SpO2) >90%, and arterial partial pressure of carbon dioxide (PaCO2) maintained at 35–45 mmHg. Patients with acute respiratory distress syndrome (ARDS) were given a lung-protective ventilation strategy, with a small tidal volume (4–8 mL/kg) to ensure that the plateau pressure was less than 30 cmH2O. Light sedation was maintained during MV [Richmond Agitation and Sedation Scale (RASS) score: −1 to +1]. Patients in the experimental group (the EDP group) (n=27) were treated with EDP (manufactured by Arahelio Biotechnology Developing Guangzhou Co., Ltd., Guangzhou, China) in addition to routine treatment. The pacing electrode was pasted on the outer third of the lower end of the sternocleidomastoid muscle, and the auxiliary electrode was placed in the second intercostal space of the midclavicular line. Due to individual differences, it was necessary to adjust the best position by measuring the change of diaphragm movement by ultrasound after electrical stimulation. The position with the greatest movement of the diaphragm was considered the best position for treatment and this position was maintained thereafter. If tolerable, the treatment intensity increased from low to high as follows: pacing was 10 min/time, the frequency was 40 Hz/30 min/time, 2 times/day, 5 days/week until patients were successfully weaning, died, transferred out to the ICU, or after a full 28 days of therapy.
All included patients were given standardized weaning protocols. The weaning criteria were assessed by the same operator who was blinded to the study design. Weaning failure was defined as repeated tracheal intubation within 48 hours or failure of the SBT during the weaning process (14,15), with the patient requiring invasive/non-invasive assisted ventilation or subsequent death. Patients on planned MV at night were not defined as failure to wean according to local medical practice. The types of weaning can be divided into the following: (I) simple weaning, where the patient is successfully weaned at the first SBT and extubated; (II) difficult weaning, which requires up to 3 SBT attempts or up to 7 days after the first SBT to successfully wean; and (III) prolonged weaning, requiring more than 3 SBT attempts or more than 7 days after the first SBT to successfully wean the patient (15).
Diaphragm thickness at the end of inspiration (DTei) and diaphragm thickness at the end of expiration (DTee) were evaluated using a GE Venue 50 ultrasound system (Venue 50, General Electric Company, Boston, MA, USA). The patient was placed in a supine position with the head of the bed raised 30°. The right midaxillary line was taken and placed vertically on the chest wall with the ultrasound linear probe. Starting from the 8th to 9th intercostal space, the diaphragmatic attachment was slid upwards and the diaphragm thickness was measured, as well as the DTei and the DTee. The DTF was calculated. To measure the DE, the M mode was used with the convex array probe placed under the ribs between the midclavicular and midaxillary lines.
Observation indicators
The DE was measured and the DTF was calculated according to the following formula: DTei and DTee were measured respectively, and DTF = (DTei − DTee)/DTee × 100% was calculated.
On one hand, the DE was recorded on days 7, 14, 21 and at the time of attempting SBT after treatment, and the corresponding DTF was calculated. The values recorded on day 7 after treatment were used as the clinical baseline for this study. On the other hand, the DMV, ILOS, and average survival time were recorded. Among them, DE is the primary outcomes, the others are secondary outcomes.
Statistical analysis
Using success weaning ratio derived from the prior researches and our pilot experiment, 68.8% for the control group, 86.8% for the EDP group, a sample of 80 patients for each group was estimated to provide 80% power to detect a significant difference with bilateral ɑ=0.05. The Shapiro-Wilk test was used for normality test and the Levene test was used for homogeneity of variance test. Measurement data that conform to normal distribution are expressed as mean ± standard deviation. The t-tests were used to compare the means of two independent samples and the Satterthwaite corrected t-test was used when the two groups showed heterogeneity of variance. If the data it did not conform to normal distribution, the median and interquartile range were used, and two independent samples were compared using rank sum test. Spearman’s nonparametric rank correlation test was used for correlation analysis. Generalized estimating equations (GEEs) was used for analyzing the repeated measures. To be specific, DE and DTF were measured at the days 7, 14, and 21, respectively, and multiple outcome data were obtained. We set DTF at day 21 as the primary outcome, while for data at other time points, we set it as a secondary outcome. The Kaplan-Meier method was used for survival analysis and compared with the log-rank test. The receiver operating characteristic (ROC) curve was used to evaluate the efficacy of ultrasound derived measurements of DTF-day 14 in predicting simple weaning. The inspection level was set to α=0.01 and bilateral P<0.05 was considered statistically significant. All statistical analyses were performed using the BMI SPSS 25 software and GraphPad Prism 8.0.1 software.
Results
The characteristics of the patients
A total of 51 patients who underwent MV in the ICU of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University from September 2019 to December 2020 were enrolled and randomly divided into an experimental group (n=27) and a control group (n=24) using a random number generator (Figure S1). The mean follow-up time was 1 year, and the follow-up was terminated in September 2021. The clinical data of the patients are shown in Table 1. There were no significant differences in age, gender, BMI, Acute Physiology, Age and Chronic Health Evaluation II (APACHE II) score, serum lactate concentration (Lac), arterial partial pressure of oxygen (PaO2), PaCO2, oxygenation index and oxygen inhalation concentration between the experimental group and the control group at the time of enrollment (P>0.05).
Table 1
| Characteristics | EDP group (n=27) | Control group (n=24) | P value |
|---|---|---|---|
| Age (years) | 60.04±13.47 | 66.33±14.45 | 0.114 |
| Female | 19 | 11 | 0.366 |
| BMI (kg/m2) | 22.06 (19.81 to 23.07) | 21.14 (19.56 to 22.26) | 0.199 |
| APACHE II (points) | 22.26±5.43 | 23.29±8.15 | 0.602 |
| Lac (mmol/L) | 2.10 (1.40 to 2.50) | 2.40 (1.85 to 2.90) | 0.192 |
| PaO2 (kPa) | 14.32 (12.45 to 21.75) | 15.52 (11.29 to 20.10) | 0.651 |
| PaCO2 (kPa) | 4.67 (4.03 to 5.24) | 4.62 (3.85 to 5.19) | 0.873 |
| PaO2/FiO2 (points) | 40.63±18.08 | 37.53±15.10 | 0.508 |
| FiO2 (%) | 0.50 (0.40 to 0.50) | 0.48 (0.40 to 0.80) | 0.466 |
Data are expressed as mean ± standard deviation, n, or median (range). P<0.05 was considered statistically significant. BMI, body mass index; APACHEII, Acute Physiology, Age and Chronic Health Evaluation II; Lac, serum lactate concentration; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; PaO2/FiO2, oxygenation index; FiO2, fraction of inspired oxygen; EDP, external diaphragmatic pacing.
A comparison of the diaphragm contraction between the EDP group and the control group
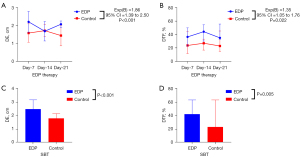
The DE and the DTF were measured in both groups after 1, 2, and 3 weeks of therapy. Both the DE and DTF [exp(B) =1.86, 95% confidence interval (CI): 1.39 to 2.50, P<0.001; exp(B) =1.35, 95% CI: 1.05 to 1.76, P=0.022, respectively; Table 2) of patients in the EDP group were significantly enhanced compared with that in the control group (Figure 1A,1B). Until the time of the SBT, both DE and DTF were improved significantly compared to that of the controls (P<0.001 and P=0.005, respectively; Figure 1C,1D).
Table 2
| Variables | DE | DTF | |||||
|---|---|---|---|---|---|---|---|
| B (95% CI) | Exp(B) (95% CI) | P | B (95% CI) | Exp(B) (95% CI) | P | ||
| (Intercept) | 1.59 (1.39, 1.79) | 4.90 (4.01, 5.99) | <0.001 | 0.26 (0.16, 0.35) | 1.30 (1.18, 1.42) | <0.001 | |
| Group | |||||||
| EDP vs. control | 0.62 (0.33, 0.92) | 1.86 (1.39, 2.50) | <0.001 | 0.30 (0.04, 0.56) | 1.35 (1.05, 1.76) | 0.022 | |
| Time | |||||||
| day-14 vs. day-7 | 0.06 (−0.22, 0.35) | 1.06 (0.80, 1.41) | 0.670 | 0.06 (−0.05, 0.17) | 1.06 (0.95, 1.19) | 0.271 | |
| day-21 vs. day-7 | −0.23 (−0.58, 0.12) | 0.80 (0.56, 1.13) | 0.204 | 0.01 (−0.08, 0.09) | 1.01 (0.92, 1.10) | 0.906 | |
| Group × time | |||||||
| EDP vs. day-14 | −0.48 (−0.84, −0.11) | 0.62 (0.43, 0.89) | 0.010 | −0.23 (−0.50, 0.05) | 0.80 (0.60, 1.05) | 0.107 | |
| EDP vs. day-21 | 0.14 (−0.53, 0.80) | 1.15 (0.59, 2.22) | 0.689 | −0.14 (−0.39, 0.11) | 0.87 (0.68, 1.12) | 0.273 | |
P<0.05 was considered statistically significant. GEE, generalized estimating equation; DE, diaphragm excursion; DTF, diaphragm thickening fraction; EDP, external diaphragmatic pacing.
EDP in mechanically ventilated patients effectively reduce the DMV
To investigate the curative effect of EDP treatment, the DMV, ILOS, and the proportion of simple weaning were analyzed. The DMV was significantly different between the groups, with the DMV of the control group being 17.50 (11.25–26.50) days and the DMV of the EDP group being 12.00 (9.00–21.00) days (P=0.026; Table 3). Furthermore, the proportion of simple weaning was higher in the EDP group compared to the control group (P<0.001; Table 3). However, the ILOS was not statistically different between the two groups (P=0.117; Table 3). More exactly, under difficult weaning conditions, 2 weeks of EDP treatment significantly improved the DTF and the DE in response to the SBT compared to patients without EDP treatment (P=0.032 and P=0.013, respectively; Figure 2A,2B). Thus, the ROC curve was constructed to determine the ability of diaphragmatic function to predict the weaning condition. The area under the curve (AUC) of the DTF ROC curve after 2 weeks of EDP treatment was 0.80 (95% CI: 0.56 to 1.00, P=0.062) and the cut-off was 44.42% (Figure 3).
Table 3
| Outcomes | EDP group (n=27) | Control group (n=24) | P value |
|---|---|---|---|
| Simple weaning (%) | 62.96 | 12.50 | <0.001 |
| DMV (days) | 12.00 (9.00 to 21.00) | 17.50 (11.25 to 26.50) | 0.026 |
| ILOS (days) | 14.00 (10.50 to 25.50) | 22.00 (13.50 to 27.50) | 0.117 |
Data are presented as % or median (range). P<0.05 was considered statistically significant. EDP, external diaphragmatic pacing; DMV, duration of mechanical ventilation; ILOS, intensive care unit length of stay.


The correlation between diaphragmatic function and Lac and respiratory parameters
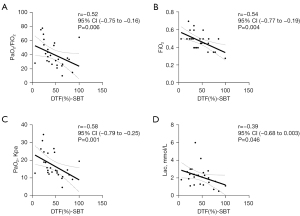
To explore the possible mechanisms by which EDP may improve diaphragmatic function, clinical data were collated and the correlation with DE and DTF was analyzed. By the time SBTs could be performed, the DTF was negatively associated with PaO2/fraction of inspired oxygen (FiO2) (r=−0.52; 95% CI: −0.75 to −0.16; P=0.006), FiO2 (r=−0.54; 95% CI: −0.77 to −0.19; P=0.004), PaO2 (r=−0.58; 95% CI: −0.79 to −0.25; P=0.001) and Lac (r=−0.39; 95% CI: −0.68 to 0.003; P=0.046) (Figure 4). The results showed that improvements in diaphragmatic function could improve gas exchange, which is closely related to increased oxygen consumption.
Mechanically ventilated patients treated with EDP had a longer average survival time
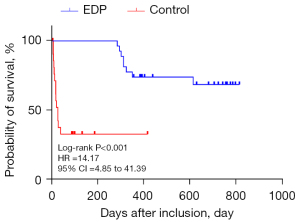
Discharged patients were followed up and observed to determine their survival time. As of September 2021, the average survival time of mechanically ventilated patients with conventional symptomatic treatment was 314.68±78.00 days compared to 586.77±67.34 days for patients on combined conventional and EDP treatment, suggesting that the average survival time of EDP patients after charge was significantly longer than that of control patients (P<0.001, Figure 5). It is noteworthy that complications of EDP can including shoulder discomfort, abdominal pain, and upper airway obstruction. However, none of these complications were observed in our study cohort.
Discussion
Diaphragm pacing stimulates the phrenic nerve by artificially simulating physiological discharges through electrical stimulation (16), causing the diaphragm to contract continuously and regularly. Based on the placement of electrodes, it can be divided into implanted diaphragm pacing (IDP) and EDP (17,18). While most previous studies have focused on the role of IDP, to the best of our knowledge, the current study is the first to examine the effects of EDP in mechanically ventilated patients. Specifically, the effect of EDP on the success rate of weaning, the DMV, and the ILOS were assessed. Consistent with our predictions, mechanically ventilated patients in the EDP-treated group showed elevated DE and DTF, significantly decreased weaning time, a higher proportion of simple weaning, and a longer mean survival time after discharge. In the case of difficult weaning, the DE at SBT attempt and the DTF after 2 weeks of EDP treatment were both improved compared with the control group. Moreover, the DTF at the time of SBT was negatively correlated with FiO2, PaO2, PaO2/FiO2, and Lac. The ROC curve indicated that DTF after 2 weeks of EDP treatment may be an independent predictor of simple weaning.
Ultrasound technology is widely used, and increasingly, novel applications are being implemented, such as bedside lung ultrasound in the ICU (19). Some indicators of diaphragm function can track dynamic changes in diaphragm size and function over time, evaluate diaphragm dysfunction, and predict successful weaning, such as DE and DTF (20). Multiple studies have reported that certain measures of diaphragm function can predict successful weaning from MV, with DE of 11–14 mm and DTF of 30–36% being the most sensitive and specific indicators (19,21-23). By analyzing the ROC curve to predict the weaning situation, it was determined that DTF after 2 weeks of treatment may be an independent predictor of simple weaning.
This study was a prospective, randomized, controlled study on the use of EDP in mechanically ventilated patients. The innovation of this study is reflected in the following aspects. First, the mechanically ventilated critically ill patients, including those with chronic obstructive pulmonary disease, pulmonary infection, sepsis, and ARDS, have not been previously examined in other clinical studies. Prior investigations have focused on the effect of the diaphragm pacemaker in patients with congenital central hypoventilation syndrome (CCHS), ALS, spinal injury, and quadriplegia (24,25). Second, the current investigation demonstrated that DTF is negatively correlated with FiO2 and PaO2/FiO2, which may be caused by the increased oxygen consumption as a result of increased diaphragm movement, suggesting that improvements in diaphragm function can improve gas exchange. Finally, this study showed that the average survival time after discharge of mechanically ventilated patients receiving EDP treatment is longer than that of those who did not receive EDP therapy, suggesting that the increase in DE and DTF after EDP treatment can improve diaphragm function, which can benefit the prognosis of mechanically ventilated patients.
This study was a single-center study with a small sample size and further large-scale, multi-centered trials should be conducted to verify these results. Due to the differences in disease severity at the time of admission to the experimental group, the parameters of MV differed. In addition, to ensure the diaphragm ultrasound was conducted by the same operator, ultrasounds prior to the experiment were not performed. The mechanically ventilated patients included in this study presented with conditions including chronic obstructive pneumonia, pulmonary infection, ARDS, and sepsis, and stratified analyses should be performed to determine the correlation between the severity of disease and patient outcome following EDP therapy.
MV is currently a “double-edged sword” in critical care medicine. While saving the lives of critically ill patients, it may also result in some adverse complications, such as ventilator-associated pneumonia and diaphragmatic disuse atrophy. Therefore, determining the optimal DMV is crucial to the long-term survival of patients and is a research hotspots in critical care medicine (26).
Conclusions
This investigation demonstrated that EDP treatment can effectively reduce the weaning time and increase the proportion of simple weaning in mechanically ventilated patients. The DTF after 2 weeks of EDP treatment was identified as an independent predictor of simple weaning, and may be useful in the long-term prognosis of mechanically ventilated critically ill patients after weaning.
Acknowledgments
Funding: The study was supported by National Natural Science Foundation of Guangdong Province (No. 2022A1515011248).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4145/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4145/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4145/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4145/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Signed informed consent forms were obtained from all enrolled patients and their families. This study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. 2019-23).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pu L, Zhu B, Jiang L, et al. Weaning critically ill patients from mechanical ventilation: A prospective cohort study. J Crit Care 2015;30:862.e7-13. [Crossref] [PubMed]
- Stieff KV, Lim F, Chen L. Factors Influencing Weaning Older Adults From Mechanical Ventilation: An Integrative Review. Crit Care Nurs Q 2017;40:165-77. [Crossref] [PubMed]
- Powers SK, Wiggs MP, Sollanek KJ, et al. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 2013;305:R464-77. [Crossref] [PubMed]
- Evans D, Shure D, Clark L, et al. Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: study protocol for a randomized trial. Trials 2019;20:60. [Crossref] [PubMed]
- Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327-35. [Crossref] [PubMed]
- Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 2013;188:213-9. [Crossref] [PubMed]
- Umbrello M, Formenti P, Longhi D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 2015;19:161. [Crossref] [PubMed]
- Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364-71. [Crossref] [PubMed]
- Lee EP, Hsia SH, Hsiao HF, et al. Evaluation of diaphragmatic function in mechanically ventilated children: An ultrasound study. PLoS One 2017;12:e0183560. [Crossref] [PubMed]
- Dres M, Demoule A. Monitoring diaphragm function in the ICU. Curr Opin Crit Care 2020;26:18-25. [Crossref] [PubMed]
- Maffiuletti NA, Roig M, Karatzanos E, et al. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med 2013;11:137. [Crossref] [PubMed]
- Monden KR, Coker J, Charlifue S, et al. Long-Term Follow-Up of Patients With Ventilator-Dependent High Tetraplegia Managed With Diaphragmatic Pacing Systems. Arch Phys Med Rehabil 2022;103:773-8. [Crossref] [PubMed]
- Kerwin AJ, Zuniga YD, Yorkgitis BK, et al. Diaphragm pacing improves respiratory mechanics in acute cervical spinal cord injury. J Trauma Acute Care Surg 2020;89:423-8. [Crossref] [PubMed]
- Kerwin AJ, Diaz Zuniga Y, Yorkgitis BK, et al. Diaphragm pacing decreases hospital charges for patients with acute cervical spinal cord injury. Trauma Surg Acute Care Open 2020;5:e000528. [Crossref] [PubMed]
- Burns KEA, Rizvi L, Cook DJ, et al. Ventilator Weaning and Discontinuation Practices for Critically Ill Patients. JAMA 2021;325:1173-84. [Crossref] [PubMed]
- Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, et al. Diaphragmatic dysfunction. Pulmonology 2019;25:223-35. [Crossref] [PubMed]
- Hannan LM, De Losa R, Romeo N, et al. Diaphragm dysfunction: a comprehensive review from diagnosis to management. Intern Med J 2021; Epub ahead of print. [Crossref] [PubMed]
- Baskaralingam A, Nicod L, Manzoni R. Diaphragmatic paralysis and paresis Rev Med Suisse 2020;16:1646-51. review. [Crossref] [PubMed]
- Ferrari G, De Filippi G, Elia F, et al. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 2014;6:8. [Crossref] [PubMed]
- Turton P. ALAidarous S, Welters I. A narrative review of diaphragm ultrasound to predict weaning from mechanical ventilation: where are we and where are we heading? Ultrasound J 2019;11:2. [Crossref] [PubMed]
- Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011;39:2627-30. [Crossref] [PubMed]
- Jiang JR, Tsai TH, Jerng JS, et al. Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest 2004;126:179-85. [Crossref] [PubMed]
- DiNino E, Gartman EJ, Sethi JM, et al. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014;69:423-7. [Crossref] [PubMed]
- Vashisht R, Chowdhury YS. Diaphragmatic Pacing. In: StatPearls. Treasure Island: StatPearls Publishing, 2022.
- Hill TM, Onugha O. Diaphragmatic Pacing: Is There a Benefit? Surg Technol Int 2019;35:265-70. [PubMed]
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033-56. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


