
Pulmonary rehabilitation ameliorates regional lung function in chronic obstructive pulmonary disease: a prospective single-arm clinical trial
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable chronic respiratory disease characterized by persistent airflow restriction. Its incidence rate and mortality rate have been at a high level for a long time, and there is a trend of continuous rise (1). Currently, COPD is one of the top three causes of death worldwide; 90% of these deaths occur in low- and middle-income countries (2). The disease is recurrent and incurable, which seriously threatens health-related quality of life (3). Although conventional drug treatment can relieve the symptoms of dyspnea, improve lung function, and reduce exacerbations to a certain extent, it has little effect on the progression of COPD.
Pulmonary rehabilitation (PR) is an important strategy in the management of patients with stable COPD. It is widely recognized as a comprehensive intervention measure of nonpharmacologic therapy (4). Long term rehabilitation, maintenance strategies following rehabilitation, and the incorporation of education and strength training in PR are beneficial to COPD patients (5). Moreover, a strengthening exercise program for both the general extremities and respiratory muscles has been included in therapeutic recommendations, which not only improve physical exercise, but also decrease exercise-induced oxidative stress damage (6). Many studies have shown that core components of PR, including exercise training combined with disease-specific education and self-management interventions, can help COPD patients alleviate shortness of breath, improve quality of life, and reduce mortality and the number of readmissions. At present, PR is ranked as one of the most cost-effective intervention strategies to treat COPD (4,7,8). Therefore, in order to facilitate the recovery of COPD, it is particularly imperative to encourage patients to carry out corresponding rehabilitation training when they are in a stable stage (9).
The full medical assessment of patients with COPD requires the use of medical imaging techniques and a pulmonary function test (PFT). Conventional radiological methods of chest radiography and computed tomography (CT) are preferentially used to visualize the morphological structural changes in the lung tissues, but these examinations are carried out discontinuously and expose patients to radiation (10). Spirometry plays an important role in evaluating the treatment of COPD, but it is derived from the measurement taken at the mouth, so it can only provide the global, average lung function information of patients (11). Since many lung diseases, including COPD, are characterized by regionally dissimilar evolution of the underlying morphological and pathophysiological lung changes, it is essential to assess regional lung function in those patients, which cannot be achieved by conventional detection equipment (12).
Electrical impedance tomography (EIT) is a novel bedside detection medical imaging technology (13). It provides real-time images of air distribution and reflects the dynamic changes that cannot be captured by traditional chest X-ray (14). It has been used to detect regional airflow limitations, which is useful in patients with obstructive lung diseases (11,15,16). However, there have been no studies evaluating the effect of PR on regional ventilation and lung functions in COPD using EIT. Although a large number of guidelines and studies have shown that PR can benefit COPD, there is no universally recognized standard for PR (17). Therefore, the aim of the present study was to investigate the effects of short-term, high-intensity PR on the regional lung ventilation in stable COPD patients using EIT. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3597/rc).
Methods
Study design
This was a single-center, prospective single-arm clinical trial in which participants were systematically evaluated to monitor the effectiveness of 2-week PR treatment on regional lung function. This study was approved by the Medical Ethics Committee of the Xijing Hospital of Air Force Medical University (No. KY20212122-F-1) and all participants signed written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Setting and participants
Participants were recruited from the Xijing Hospital of Air Force Medical University from January 2021 to February 2022. The participants inclusion criteria were as follows: (I) COPD, as diagnosed by spirometry, with a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <0.7; (II) in a stable period; (III) aged between 40 and 80 years old; and (IV) with normal understanding, clear consciousness, and willing to sign informed consent to the research process. The exclusion criteria were as follows: (I) hospitalization; (II) malignant tumors, autoimmune diseases, systemic infectious diseases, and vital organ dysfunction; (III) pleural disease or thoracic deformity; and (IV) contraindications to PR or other reasons that rendered them unsuitable for the study. Because most patients with COPD have complications and are prone to co-infection, they were excluded from the study according to strict exclusion criteria. Moreover, this study is an exploratory clinical trial, which also refers to the sample size of previous studies about EIT (13,15,16). These may cause our sample size to be small.
Interventions
In addition to routine treatment and health education, all participants also used the respiratory rehabilitation training instrument (TKBR-01, Tianjian Medical Instrument, Wuxi, China) to carry out respiratory muscle training and vibration expectoration. Since the existing guidelines and most published studies to date do not have uniform requirements on the frequency, timing, and method of PR (17), our study chose short-term, high-intensity, and center-based PR (18,19): 30 minutes per training session, once a day, and continuous training for 2 weeks.
Respiratory muscle training: the patient held their breath for 2 seconds after fast and deep inhalation, and then slowly exhaled the gas; the process took about 8 seconds each time. The process was repeated 5 times for each group, with an interval of 5–10 seconds after each group of training. Initially, the whole program was conducted for a total of 4 groups. In further PR, 1–2 additional groups were added as appropriate. If the patient did not feel tired after 6 consecutive groups of training at the same intensity, the intensity was increased appropriately.
Vibration expectoration: the patient kept the expectorator upright and forced expiration was performed with the aim of shaking the small ball in the expectorator. The process was repeated 3×3 times. After each group was completed, patients were encouraged to cough and expectorate, increasing expiratory resistance as appropriate.
Measurements
The participants were evaluated for symptoms, PFT (MasterScreen PFT system, Jaeger, Hoechberg, Germany) and EIT (PulmoVista 500, Dräger Medical, Lübeck, Germany) before and after the PR. The EIT electrode band was placed at the 4th intercostal spaces for male patients, and at the 5th intercostal spaces for female patients. The electrode belt size was selected according to the patient’s chest circumference. We conducted EIT and PFT simultaneously in the morning before and after the 2-week PR program. The specific EIT examination was conducted as previously reported (20), and PFT was conducted following the American Thoracic Society/European Respiratory Society (ATS/ERS) standards (21). The patients were tested at least 3 times for each measurement, and the most qualified test results were taken.
To evaluate the symptoms of participants, the modified Medical Research Council (mMRC) dyspnea scale and COPD assessment test (CAT) score were applied. The PFT parameters included FEV1, FVC, slow vital capacity (SVC), peak expiratory flow (PEF), FEV1 predicted percentage (%pred), and FEV1/FVC.
Regional lung function was assessed using EIT. The parameter setting and data reconstruction method of EIT were conducted as previously reported (20). Spatial ventilation heterogeneity included the coefficient of variation (CV) of the pixel values of FEV1/FVC, FEV1, FVC, and PEF. The CV was calculated as the ratio of the standard deviation (SD) to the mean pixel value of each parameter (11). Temporal ventilation heterogeneity included the pixel expiration time needed to expire 90% (t90), 75% (t75), 50% (t50), and 25% (t25) of the respective pixel FVC.
Statistical analysis
Data are expressed as means ± SD. Differences in outcomes were compared using the Wilcoxon test for paired samples due to the small number of subjects. Statistical significance was accepted if the P value was <0.05 (two-tailed). The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
Baseline characteristics
The study design is shown in Figure 1. A total of 34 patients who met the criteria were included in this study. Of these, 5 were excluded due to refusal to participate (n=3) and other reasons (n=2); 5 patients failed to complete the examinations because of acute exacerbation (n=2) and loss of interest in the study (n=3). In total, 24 participants completed the PR (Figure 1). At baseline, 70.8% of the patients were male, and their age was 64.4±6.30 years. Some 66.7% of the patients had a smoking history, with an average duration of 22.9 years. All participants received bronchodilator treatment, and more than half of the cohort received dual bronchodilator treatment. The baseline characteristics of these patients are shown in Table 1.
Table 1
| Variable | COPD (n=24) |
|---|---|
| Demographics | |
| Gender, males, n (%) | 17 (70.8) |
| Age (years) | 64.4±6.30 |
| Height (cm) | 165.0±9.24 |
| Weight (kg) | 64.9±12.69 |
| BMI (kg/m2) | 23.6±2.95 |
| Ex-smokers, n (%) | 16 (66.7) |
| Pack-years (smoker/ex) | 22.9±18.99 |
| Medication, n (%) | |
| LAMA | 6 (25.0) |
| LABA | 5 (20.8) |
| LABA + LAMA | 13 (54.2) |
| GOLD classification, n (%) | |
| I | 1 (4.2) |
| II | 10 (41.7) |
| III | 5 (20.8) |
| IV | 8 (33.3) |
| GOLD category, n (%) | |
| A | 4 (16.7) |
| B | 8 (33.3) |
| C | 3 (12.5) |
| D | 9 (37.5) |
| Pulmonary function | |
| FEV1 (L) | 1.2±0.65 |
| FEV1 (%pred) | 47.5±23.03 |
| FVC (L) | 2.2±0.95 |
| FEV1/FVC (%) | 57.3±14.85 |
| SVC (L) | 2.4±1.05 |
| PEF (L/s) | 3.3±2.00 |
Enumeration data are presented as n (%), and measurement data are presented as mean ± SD unless otherwise stated. COPD, chronic obstructive pulmonary disease; BMI, body mass index; LAMA, long-acting muscarinic antagonists; LABA, long-acting beta2-agonist; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SVC, slow vital capacity; PEF, peak expiratory flow.
Effects of PR on symptoms
There was significant improvement in the symptoms of COPD patients after the 2-week PR treatment. The mMRC dyspnea scale and the CAT score were significantly lower compared to those measured before the PR treatment (2.3±1.17 vs. 2.1±0.93, P=0.034; and 15.0±7.18 vs. 10.9±6.06, P<0.001, respectively) (Figure 2).

Effects of PR on pulmonary function
There was no significant difference between SVC and FEV1/FVC in pulmonary function after 2 weeks of rehabilitation treatment (2.5±1.11 vs. 2.6±0.98 L, P=0.279; and 58.1%±18.19% vs. 59.1%±17.63%, P=0.290, respectively). However, FVC, FEV1, FEV1 %pred, and PEF were significantly better than they were pre-rehabilitation (2.1±0.86 vs. 2.3±0.90 L, P=0.018; 1.2±0.65 vs. 1.4±0.66 L, P=0.001; 46.8%±23.16% vs. 51.4%±24.41%, P<0.001; and 3.1±1.80 vs. 3.8±2.23 L/s, P=0.005, respectively) (Figure 3).

Effects of PR on regional lung function
Spatial ventilation heterogeneity
The CV of FEV1/FVC decreased significantly after the 2-week PR (0.26±0.161 vs. 0.17±0.077, P=0.002), and the range of spatial heterogeneity was narrower than that before the treatment. However, there was no significant difference in the CV of FEV1, FVC, and PEF (Figure 4).

Temporal ventilation heterogeneity
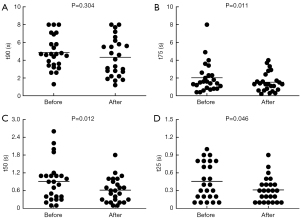
Histograms of pixel t90, t75, t50, and t25 indicate the heterogeneity of regional lung emptying during the forced full expiration. Compared with pre-rehabilitation, the degree of temporal heterogeneity in lung emptying of participants was lower (the initial frequency distribution of pixel was very broad) (Figure 5), and the center expiration times of t75, t50, and t25 significantly reduced after intervention (Figure 6). Thus, the expiration times of patients with COPD was shorter and more uniform after PR (the pixel values were shifted to the left).

Discussion
This study evaluated the impact of PR in stable COPD on regional lung ventilation. For the first time, EIT was introduced to assess the regional lung function of COPD patients who underwent PR. We found that both spatial and temporal regional ventilation in COPD improved after the 2-week PR.
Effects of PR on symptoms
This prospective study demonstrated a significant improvement in the symptoms of COPD assessed by the mMRC dyspnea scale and CAT score after 2-week of PR program. The center-based PR in this study is more standardized than other home-based PR. We found that PR can improve the symptoms of COPD patients, especially in reducing dyspnea and improving exercise endurance, which is consistent with the findings of many clinical trials (5,22). A previous study found that exercise training can partially reverse the shift towards glycolytic fibers in COPD patients (23). High intensity PR for 2 weeks induced increased protein expression and mitochondrial enzyme activity, leading to enhanced oxidative capacity of the muscles (19). Improved tissue oxidative activity of the muscles during exercise facilitates higher gains in exercise tolerance and is associated with reduced ventilatory drive to breathe, which can reduce dyspnea sensations (22). After 2 weeks of high-intensity PR, the symptoms of COPD patients will be improved to some extent.
Effects of PR on pulmonary function
We found that PR can improve FVC, FEV1, FEV1 %pred, and PEF in COPD, but there was no significant difference in FEV1/FVC and SVC. Leelarungrayub et al. reported that respiratory muscle training has a significant improvement in patients with COPD, especially FEV1 and FEV1 %pred (6); our study performed a fast deep breathing technique on patients through a respiratory muscle trainer, and the results were consistent with their findings. It has been proposed that the improvement of lung function could be a result of the implementation of fast and deep breathing, which may stimulate respiratory muscle function. Inspiratory capacity has been found to be a major contributor to endurance capacity, reflecting the operating limits for tidal volume expansion and CO2 retention during incremental exercise (22). Inspiratory muscle training increases transpulmonary pressure, inspiratory volume, and inspiratory muscle performance (24). According to the study by Ferraro et al., inspiratory muscle shortening velocity moderately increases and inspiratory muscle strength significantly improves following inspiratory muscle training, thereby enhancing pulmonary and inspiratory muscle function (25). The short training period may be the reason why there was no significant difference between FEV1/FVC and SVC.
Effects of PR on regional lung function
Characteristically, COPD involves regionally dissimilar evolution of the underlying morphological and pathophysiological lung changes (12). The use of EIT is useful for clinicians to observe changes in regional lung function over a period of time to quantify pulmonary disease progression, which may be beneficial to patients with COPD with their heterogeneous pattern of structural and functional lung damage (26). Therefore, in the present study, we adopted EIT technology to assess the regional lung function in COPD patients. Apart from the symptoms and global lung function, EIT provided us new insights to PR, with the spatial and temporal distribution of airflow limitation. Interestingly, although the global spirometry of FEV1/FVC did not improve significantly after the 2-week PR, the spatial heterogeneity of FEV1/FVC decreased significantly. In addition, the temporal heterogeneity of regional lung emptying was lower, and expiration times were shorter than pre-rehabilitation, especially in t75, t50, and t25. This is the first study to report that PR improved the regional temporal and spatial heterogeneity of COPD.
Previous studies have shown that the spatial distribution of regional volumetric lung function measures determined by EIT is heterogeneous in patients with COPD and consistently higher than that in healthy individuals (27,28). Milne et al. developed time-based features of regional ventilation using EIT measurements, which exhibited significant regional heterogeneity in COPD compared with healthy controls (29). A previous study also indicated that those with COPD had an overall increased ventilation heterogeneity and CV (26), and we found that PR can make the regional distribution of spatial and temporal ventilation show less heterogeneity than pre-rehabilitation.
The mechanism underlying the improved regional lung function in COPD patients following two-week PR is not clear. Combining the symptoms and global lung function, we speculated that PR can stimulate respiratory muscle function, improve muscle endurance and contractility, and enhance muscle regulation, hence to make regional lung ventilation more homogeneous and regional expiration time shorter after PR.
Furthermore, the majority of patients with COPD need to use bronchodilators as the basic treatment to reduce symptoms and the risk and severity of exacerbations, as well as improve their health status and exercise tolerance (17), including the patients who participated in this study. Troosters et al. proposed that bronchodilator treatment and respiratory muscle training in patients with COPD can improve exercise tolerance, but this involves different and complementary pathways (30). From a physiologic standpoint, improved muscle function after PR is associated with a consequent reduction in ventilatory requirements for a given level of exercise, leading to reduced dynamic hyperinflation (30). Bronchodilators act by altering airway smooth muscle tone and widening of the airway, which tends to reduce dynamic hyperinflation at rest and during exercise (31). It was suggested that respiratory muscle training and bronchodilators can be co-optimized, and the combined effect is greater than the algebraic sum of their single effects. Under the promotion of bronchodilators, PR can play a greater role, which could be one of the reasons for the improvement of regional lung function. Although it has been previously demonstrated that bronchodilators can improve regional lung function in patients with COPD (27), in this study, the influence of bronchodilators on the results can be excluded, because patients used bronchodilators regularly following the doctor’s advice throughout the PR. Consequently, based on the above research on the mutual optimization of bronchodilators and PR, we conclude that PR, with or without the assistance of bronchodilators, can reduce the heterogeneity of regional ventilation in COPD through its own ability, but bronchodilators can promote PR to play a greater role.
In addition, study using EIT for regional lung perfusion analysis are still in the exploratory stage (32). In the future, we can use this feature to observe whether the PR treatment to patients with COPD has an impact on their regional lung perfusion, to yield a deeper and more comprehensive understanding of PR.
Limitations
This study was designed as a prospective observational study, but the small sample size may have led to unstable results. Therefore, it is necessary to expand the sample size for further research. Moreover, most of the patients with COPD included in this study were moderate and severe, and mild patients should be appropriately added in the follow-up. As the period of PR in this study was short, whether a longer research cycle will benefit patients more remains to be elucidated. At the same time, because there are few studies on the PR in patients with acute acerbation, whether such patients can benefit from PR remains to be discussed. In addition, the study did not conduct long-term follow-up of patients, so it is impossible to comment on the duration of the impact of PR on COPD. Despite these many limitations, we believe that our study shows that PR can improve regional ventilation in patients with COPD to some extent.
Conclusions
This study suggested that PR can improve patients’ symptoms and pulmonary function, as well as temporal and spatial heterogeneity. Regional information provided by EIT may be helpful for developing more effective training mode of PR.
Acknowledgments
We would like to thank all the patients who participated in this study for their assistance.
Funding: The study was supported by Xijing Hospital Promoting Project (Nos. XJZT21CM13, 21FYFH02, 20202JSTS10).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3597/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3597/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3597/coif).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of the Xijing Hospital of Air Force Medical University (No. KY20212122-F-1) and all participants signed written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev 2009;18:213-21. [Crossref] [PubMed]
- Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis 2019;23:1131-41. [Crossref] [PubMed]
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14-23. [Crossref] [PubMed]
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [Crossref] [PubMed]
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. [Crossref] [PubMed]
- Leelarungrayub J, Puntumetakul R, Sriboonreung T, et al. Preliminary study: comparative effects of lung volume therapy between slow and fast deep-breathing techniques on pulmonary function, respiratory muscle strength, oxidative stress, cytokines, 6-minute walking distance, and quality of life in persons with COPD. Int J Chron Obstruct Pulmon Dis 2018;13:3909-21. [Crossref] [PubMed]
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;CD003793. [Crossref] [PubMed]
- Gloeckl R, Schneeberger T, Jarosch I, et al. Pulmonary Rehabilitation and Exercise Training in Chronic Obstructive Pulmonary Disease. Dtsch Arztebl Int 2018;115:117-23. [Crossref] [PubMed]
- Spruit MA, Wouters EFM. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology 2019;24:838-43. [Crossref] [PubMed]
- Frerichs I, Lasarow L, Strodthoff C, et al. Spatial Ventilation Inhomogeneity Determined by Electrical Impedance Tomography in Patients With Chronic Obstructive Lung Disease. Front Physiol 2021;12:762791. [Crossref] [PubMed]
- Lasarow L, Vogt B, Zhao Z, et al. Regional lung function measures determined by electrical impedance tomography during repetitive ventilation manoeuvres in patients with COPD. Physiol Meas 2021;42:015008. [Crossref] [PubMed]
- Sang L, Zhao Z, Lin Z, et al. A narrative review of electrical impedance tomography in lung diseases with flow limitation and hyperinflation: methodologies and applications. Ann Transl Med 2020;8:1688. [Crossref] [PubMed]
- Zhang N, Jiang H, Zhang C, et al. The influence of an electrical impedance tomography belt on lung function determined by spirometry in sitting position. Physiol Meas 2020;41:044002. [Crossref] [PubMed]
- Davies P, Silvestre C. Electrical impedance tomography in clinical use: Unnecessary technology or a unique angle in respiratory monitoring? Pediatr Pulmonol 2020;55:845-6. [Crossref] [PubMed]
- Vogt B, Pulletz S, Elke G, et al. Spatial and temporal heterogeneity of regional lung ventilation determined by electrical impedance tomography during pulmonary function testing. J Appl Physiol (1985) 2012;113:1154-61. [Crossref] [PubMed]
- Muller PA, Mueller JL, Mellenthin M, et al. Evaluation of surrogate measures of pulmonary function derived from electrical impedance tomography data in children with cystic fibrosis. Physiol Meas 2018;39:045008. [Crossref] [PubMed]
- Cornelison SD, Pascual RM. Pulmonary Rehabilitation in the Management of Chronic Lung Disease. Med Clin North Am 2019;103:577-84. [Crossref] [PubMed]
- Rutkowski S, Rutkowska A, Kiper P, et al. Virtual Reality Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Int J Chron Obstruct Pulmon Dis 2020;15:117-24. [Crossref] [PubMed]
- Jacobs RA, Flück D, Bonne TC, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol (1985) 2013;115:785-93. [PubMed]
- Frerichs I, Amato MB, van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017;72:83-93. [Crossref] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-e88. [Crossref] [PubMed]
- Alexiou C, Ward L, Hume E, et al. Effect of interval compared to continuous exercise training on physiological responses in patients with chronic respiratory diseases: A systematic review and meta-analysis. Chron Respir Dis 2021;18:14799731211041506. [Crossref] [PubMed]
- De Brandt J, Spruit MA, Derave W, et al. Changes in structural and metabolic muscle characteristics following exercise-based interventions in patients with COPD: a systematic review. Expert Rev Respir Med 2016;10:521-45. [Crossref] [PubMed]
- Heydari A, Farzad M. Ahmadi hosseini SH. Comparing Inspiratory Resistive Muscle Training with Incentive Spirometry on Rehabilitation of COPD Patients. Rehabil Nurs 2015;40:243-8. [Crossref] [PubMed]
- Ferraro FV, Gavin JP, Wainwright T, et al. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study. Physiol Rep 2019;7:e14076. [Crossref] [PubMed]
- Jung T, Vij N. Early Diagnosis and Real-Time Monitoring of Regional Lung Function Changes to Prevent Chronic Obstructive Pulmonary Disease Progression to Severe Emphysema. J Clin Med 2021;10:5811. [Crossref] [PubMed]
- Vogt B, Zhao Z, Zabel P, et al. Regional lung response to bronchodilator reversibility testing determined by electrical impedance tomography in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2016;311:L8-L19. [Crossref] [PubMed]
- Trenk F, Mendes L, Carvalho P, et al. Evaluation of lung ventilation distribution in chronic obstructive pulmonary disease patients using the global inhomogeneity index. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:5286-9. [Crossref] [PubMed]
- Milne S, Huvanandana J, Nguyen C, et al. Time-based pulmonary features from electrical impedance tomography demonstrate ventilation heterogeneity in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2019;127:1441-52. [Crossref] [PubMed]
- Troosters T, Maltais F, Leidy N, et al. Effect of Bronchodilation, Exercise Training, and Behavior Modification on Symptoms and Physical Activity in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018;198:1021-32. [Crossref] [PubMed]
- Kawachi S, Fujimoto K. Efficacy of tiotropium and olodaterol combination therapy on dynamic lung hyperinflation evaluated by hyperventilation in COPD: an open-label, comparative before and after treatment study. Int J Chron Obstruct Pulmon Dis 2019;14:1167-76. [Crossref] [PubMed]
- Kircher M, Elke G, Stender B, et al. Regional Lung Perfusion Analysis in Experimental ARDS by Electrical Impedance and Computed Tomography. IEEE Trans Med Imaging 2021;40:251-61. [Crossref] [PubMed]
(English Language Editor: J. Jones)



