
Messenger RNA expression profiles and bioinformatics analysis of mouse hippocampi during exercise alleviates methamphetamine dependence via mRNA profile change in hippocampi
Introduction
Methamphetamine (METH), also known as deoxyephedrine, is a highly addictive psychoactive drug. Due to its transparent white appearance resembling ice or rock sugar, METH is commonly known as ice (1). One of the most widely abused drugs in the world, METH activates the reward system of the brain and produces highly reinforcing effects that lead to abuse and dependence (2). In addition, METH abuse can bring various harmful changes to the emotional state and cognitive function of individuals, thus resulting in a decline in their physical health and quality of life (3). At present, treatment methods for METH addiction mainly focus on drug substitution therapy, cognitive behavioral therapy, health education, psychological therapy, and residential rehabilitation. Although these methods have certain efficacy, they also have some problems. The effect of drug substitution therapies is temporary due to the addictive nature of drugs, the implementation of other therapies is difficult due to poor patient compliance, and there is a lack of a standard for evaluating system efficacy (2,4). Therefore, new treatments are urgently needed for drug addicts. Recent studies have found that scientific and appropriate exercise interventions based on the above treatment methods can improve the psychological state of drug addicts, strengthen their physical fitness, and promote their physical and mental rehabilitation (5-7). Long-term regular exercise has been shown to exert a significant ameliorative effect on METH withdrawal symptoms and reduces METH relapse (4). As an important part of the limbic system, the hippocampus plays an important role in short-te memory, long-term memory, and spatial memory processing. In some previous studies, METH caused a significant decrease in the short- and long-term spatial memories of rats, and the hippocampal volume changed significantly after METH treatment, which also caused astrocyte proliferation (8). Therefore, the hippocampus is crucial to the study of the potential rewarding properties of METH and memory, as well as the recovery of psychostimulant-seeking behaviors (9). Conditioned place preference (CPP) is a classical (Pavlovian) conditioned reflex process in which one environment is paired with a drug injection and a different environment is paired with a control injection. In the context of subsequent drug and control pairings, increased preference for the drug environment is a measure of the drug-reward effect (10,11). Based on the above studies, we conclude that exercise can significantly reduce the degree of addiction to METH. We used CPP training to detect METH preference in mice. Transcriptome sequencing is a powerful technique that can be used to study the molecular changes behind differences in physiological conditions and disease progression through the identification of genes that vary significantly between sample groups. Currently, RNA sequencing (RNA-seq) transcriptome analysis is becoming increasingly popular in molecular phenotypic studies (12). It has the potential to quantify low-expression genes, revealing subtle changes in gene expression (13). Both METH and exercise may lead to gene changes. In this study, we screened differentially expressed genes (DEGs) by sequencing, which provided new ideas for future molecular phenotype studies. We explored the influence of running exercise on the METH dependency of C57BL/6J mice by establishing a METH addiction mouse model. We showed that exercise could interfere with cue memory established by the drug and the environment and reduce drug-induced CPP after withdrawal. By performing an analysis of the DEGs of hippocampal and selected functions and the candidate genes significantly related to exercise intervention, this study explored a potential new method for drug abstinence treatment. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-450/rc).
Methods
Animals
Male C57BL/6J mice (6 weeks old, 18–22 g weight) were purchased from Chongqing Tengxin Biotechnology Co., Ltd. (Chongqing, China). Before the experiment, the mice were reared in separate cages for 2 weeks for environmental adaptation. The room temperature was 22±2 ℃ with a dark/light cycle of 12 hours (light from 7:00 am to 7:00 pm) and free access to food and water (14). The animals were divided into a control group and a METH-administration group by random number method. After the METH mice model was established, the METH model group and the control group were randomly divided into an exercise group and a non-exercise group. Each group contained 10 mice. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Kunming Medical University and carried out in accordance with the relevant laws and regulations in China [ethics review approval No. kmmu20211261. Animal laboratory facility Permit Number: SYXK (Dian) K-2020-0006]. All the procedures were conducted according to the Chinese Guidelines for the Care and Use of Laboratory Animals.
Animal experimental instruments
The small animal treadmill platform (Model SA101B; Kunming Baiole Biotechnology Co., Ltd., Yunnan, China) was used to exercise the mice. The CPP experimental system (Model 2A213; Kunming Baiole Biotechnology Co., Ltd.) was composed of three compartments. The size of the CPP box was 80 cm × 30 cm × 25 cm, and the center of the box had a movable door (5 cm × 7 cm) to divide it into symmetrical spaces on both sides (30 cm × 30 cm × 25 cm). One of the inner walls was black with white stripes, with a round hole into a hollow bottom. When the partition was lifted, the mice were free to move between the two sides of the box. An infrared monitoring system recorded the length of time each mouse spent in each compartment and the number of times it crossed through the compartment.
Reagent
The drug of METH salt, which came from the Public Security Department of Yunnan Province, was dissolved in 0.9% saline to reach a concentration of 75%, then administered via an intraperitoneal (i.p.) injection of 0.2 mL to the mice at a dose of 5 mg/kg, in accordance with the literature (15,16) and the preliminary laboratory analysis. The control group was administered the same volume of saline.
Establishment of animal models
A CPP experiment is generally divided into three stages: pre-test, experiment, and test. The model was established according to the literature (15) and was the same model as that used in previous studies in our laboratory. For the pretest phase (day 1), the mice (METH + exercise group, METH + sedentary group, saline + exercise group, saline + sedentary group) were moved into the examination room 1 hour in advance and kept in darkness to reduce noise and minimize stress. Before formal training, the partition was removed, the experimental animals were placed into the experimental box and allowed to move freely for 15 minutes, with their stay time in each box recorded. After each experiment, feces and urine were removed from the mice, and the floor and walls of each compartment were wiped with 75% ethanol solution to avoid odor affecting the results. At this stage, animals can become familiar with the experimental equipment, reducing the novelty and stress, and natural preference (unconditioned place preference) can be recorded and used to select animals. To eliminate large individual differences, mice that spent more than 600 seconds in a single compartment or shuttled back and forth less than 20 times were excluded (of the 43 mice used in the current study, 3 were excluded). For adaptation training on Day 1, mice were put into the box and permitted free movement for 20 minutes to adjust to the box environment. For the training phase on days 2 to 10, the METH group of mice were injected with METH (i.p.) and placed in the drug box (black box), which was removed 30 minutes later. Mice in the saline group were intraperitoneally injected with the same volume of saline and placed in the accompanying medicine box (black box), which was removed 30 minutes later. The control experiment was conducted at the same time on the second day. The METH group and the saline group of mice were injected with control (saline) and placed in the non-accompanied drug box (black and white box). The experiment lasted for the same time as before. The experiment was alternated 4 times. For the test phase, after 8 days of training, the animals were placed on the aisle without any drug treatment, and the partitions were removed. The animals could shuttle freely between each box. The test was conducted once, and the stay time of the animals in each box was recorded. The long-stay time in the medication cabinet was significantly different from that in the control group, indicating that the mice were mentally dependent on METH. All behavioral experiments were conducted during the light phase of the light/dark cycle (7:00 am–7:00 pm).
Exercise plan
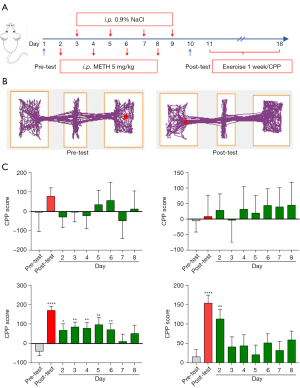
After the exercise intervention was established, the mouse model was given a moderate intensity aerobic treadmill exercise (17,18). The CPP test was conducted every day before the exercise intervention until there was no significant difference between the CPP test and the pretest score for 2 consecutive days, indicating that the CPP had disappeared. The exercise group received treadmill training at a speed of 10 meters/minute, an inclination of 0°, and an acceleration of 8 meters/minute for 45 minutes/day, lasting 7 days in total (6). All treadmill sessions were scheduled from 9:00 am to 11:30 am. The sedentary group was placed on a treadmill, but did not exercise. The sedentary group and the exercise group were tested daily for CPP (Figure 1A).
Organizational preparations
The mice were sacrificed via cervical dislocation immediately after the CPP behavior test. The whole brains were removed from their heads and placed on an ice plate. The bilateral hippocampus of each brain was carefully dissected. The mice were quickly frozen in liquid nitrogen for 1 hour and then transferred to a −80 ℃ refrigerator for preservation. Three repeat samples from each group were sent to Lianchuan Biological Co., Ltd. (Zhejiang, China) for reference transcriptome sequencing to identify DEGs.
Hippocampal transcriptome analysis
The expression levels of genes were evaluated by fragments per kilobase of transcript per million reads/fragments mapped (FPKM). The standard for identifying DEGs was an expression fold change ≥2 and a false discovery rate (FDR) lower than 0.01. A functional analysis of DEGs was performed using Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA from the hippocampus was extracted using Trizol reagent (phenol/guanidine isothiocyanate). Reverse transcription was performed according to the Goldenstar RT6 cDNA Synthesis Kit v.2 (Optimus Biotechnology Co., Ltd., Wuhan, China) protocol. Real-time qRT-PCR was performed using 2× T5 SYBR Green I Fast qPCR Mix (Optimus Biotechnology Co., Ltd., Wuhan, China). The reference gene and target gene sequence of primer 3.0 plus design of Hangzhou Bioer FQD-96A (Hangzhou Bioer Tech., Zhejiang, China) are shown in Table 1. The conditions of PCR were as follows: 95 ℃ for 30 seconds, 40 cycles of 95 ℃ for 5 seconds, 60 ℃ for 30 seconds, and 72 ℃ for 30 seconds. The melting curve analysis was executed at 95 ℃ for 15 seconds, 60 ℃ for 1 minute, and 95 ℃ for 15 seconds. The relative messenger RNA (mRNA) expression of DEGs was calculated using the 2−ΔΔCTmethod.
Table 1
| Primer number | Primer sequences | Bases | Fragment size |
|---|---|---|---|
| Beta-actin F | TGCTGTCCCTGTATGCCTCTG | 21 | 131 |
| Beta-actin R | TGATGTCACGCACGATTTCC | 20 | |
| Fos F | GTTCGTGAAACACACCAGGC | 20 | 184 |
| Fos R | GGCCTTGACTCACATGCTCT | 20 | |
| Gm12918 F | TGAGGTGCCCTACAGTGAGA | 20 | 144 |
| Gm12918 R | TGGACTTGTTTCGCCTCCTC | 20 | |
| Dagla F | CTTCGTCAAGCTGAGAGCCA | 20 | 114 |
| Dagla R | AACACTTTTAGACGGCGGGA | 20 | |
| Pip5k1c F | CGGCTCTGTCTTCTACGTCA | 20 | 125 |
| Pip5k1c R | TCCGTGGGTTCTGGTTGAGA | 20 | |
| Stxbp1 F | CACGATGGACCCCGATCATT | 20 | 240 |
| Stxbp1 R | CTTCGTAAGCACAGCGCATC | 20 | |
| Mgll F | ATCCAGAAGGACTACCCCGA | 20 | 98 |
| Mgll R | AAGTAGGTTGGCCTCTCTGC | 20 | |
| Rpl30-ps9 F | TACGTGCTGGGCTACAAACA | 20 | 81 |
| Rpl30-ps9 R | TGGACAGTTGTTGGCAAGGA | 20 | |
| Lrrc4b-1 F | TGTCAACACCCGCTACCTGA | 20 | 129 |
| Lrrc4b-1 R | CCCACCTCGATCTTTCGCAC | 20 | |
| Mapt-1 F | GTGTGGCTCGTTAGGGAACA | 20 | 174 |
| Mapt-1 R | CTGAAGGTCAGCTTGTGGGT | 20 | |
| Napg-1 F | GTCTGCAACTCGCCCCTTTT | 20 | 223 |
| Napg-1 R | ATTCCCGTCTCCTCATCTCCT | 21 | |
| Hnrnpa3-1 F | GGTGGATGCTGCAATGTGTG | 20 | 104 |
| Hnrnpa3-1 R | AATGGGCACCAGGCTTTACA | 20 | |
| Pcdhgb1 F | GCTTTTTCCAGCACCCATGA | 20 | 231 |
| Pcdhgb1 R | GCAGAACAAAGGCACCAGGA | 20 |
qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; F, forward primer, R, reverse primer.
Statistical analysis
All experiments were repeated 3 times. The CPP score was calculated by subtracting the time spent in the non-accompanied box from that spent in the accompanied box to reflect the degree of addiction of mice (19). The laboratory data were represented as the mean ± the standard error of the mean (SEM). The tests were in line with normal distribution, so the differences between the control and treatment groups were t-tested using GraphPad Prism 8.0.1 software (GraphPad Software Inc., San Diego, CA, USA). A P value of less than 0.05 was considered statistically significant.
Results
Exercise intervention accelerated withdrawal of METH-addicted mice
Male mice received an injection (i.p.) of METH for the addiction model and performed exercise intervention, and the CPP was used to detect the preference of mice. The results showed that exercise could shorten the abstinence time of the METH group of mice (Figure 1B). For the saline and saline + running group, a CPP preference was not found among the pre-test and post-test days. The CPP preference of the METH-addicted mice who underwent exercise intervention lasted to day 3, while the CPP preference of the METH-addicted mice of the sedentary group lasted until day 6 (Figure 1C). These results suggested that exercise can accelerate the recovery of METH addiction in mice.
DEG screening
The hippocampus tissue of the mice was collected, and 3 repeated samples were taken from each group. Then, RNA was extracted from the samples and used for sequencing and transcriptome analysis. There were 4 groups: Saline, Saline + Running, METH, and METH + Running. These DEGs were thoroughly screened for suitable candidate genes. Data of the DEGs were downloaded from the Lianchuan Bio-cloud platform, and Excel software (Microsoft Corp., Redmond, WA, USA) was adopted to take FPKM >0.5, ǀlog2FCǀ >0.5, and P<0.05 as the screening parameters. Of 608 genes detected by RNA-seq, DEGs (317 significant up and 291 significant down) were determined. The heat was used to exercise map showed the differential expression of genes in the hippocampal region of the 4 groups of mice, indicating that exercise could cause the gene expression of hippocampus tissues in METH-addicted mice to change (Figure 2). To further screen out genes with large differences, we took FPKM >10 as the screening criterion for further screening. There were 30 genes (14 up-regulated and 16 down-regulated) screened out in the mice with METH addiction. Since exercise alone also leads to gene changes, it was necessary to exclude the exercise-induced gene changes, regardless of addiction. The volcano map showed up-regulation and down-regulation of DEGs between the METH and METH + Running groups, and 16 prominent up- and down-regulated DEGs were noted (Figure 3A). Venn diagrams showed 3 genes overlapping between the Saline + Running, Saline and METH + Running, and METH groups, which needed to be excluded as running did not affect these genes (Figure 3B). Finally, we excluded genes with large differences among the repeated sample of the same group and screened out 12 genes that were significantly affected by METH addiction with exercise and needed further verification (Table 2). The genes Gm12918, Pcdhgb1, Dagla, Mgll, RPL30-PS9, and Fos were highly expressed, while Pip5k1c, Stxbp1, Lrrc4b, Mapt, Napg, and Hnrnpa3 were lowly expressed (Figure 4A).
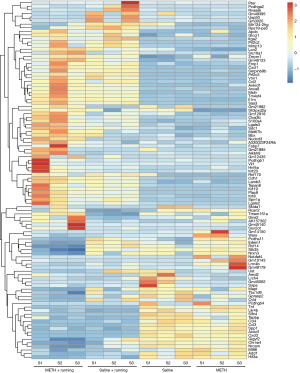
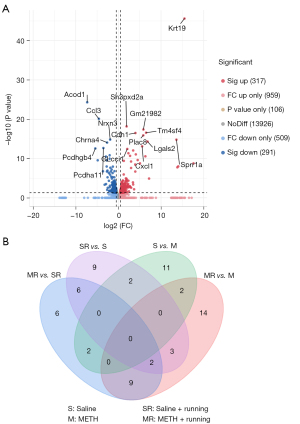
Table 2
| Gene symbol | Description | Function |
|---|---|---|
| Gm12918 | Predicted gene 12918 | Unknown |
| Pcdhgb1 | Protocadherin gamma subfamily B, 1 | These gene clusters have an immunoglobulin-like organization, suggesting that a novel mechanism may be involved in their regulation and expression. These neural, cadherin-like, cell adhesion proteins most likely play a critical role in the establishment and function of specific cell-cell connections in the brain |
| Dagla | Diacylglycerol lipase, alpha | Required for axonal growth during development and for retrograde, synaptic signaling at mature synapses (by similarity) |
| Pip5k1c | Phosphatidylinositol-4-phosphate 5-kinase, type 1 gamma | Participates in a variety of cellular processes, such as vesicle-mediated transport |
| Stxbp1 | Syntaxin binding protein 1 | May participate in the regulation of synaptic vesicle docking and fusion, possibly through interaction with GTP-binding proteins |
| Mgll | monoglyceride lipase | Hydrolyzes the endocannabinoid 2-arachidonoylglycerol, and thereby contributes to the regulation of endocannabinoid signaling, nociperception, and perception of pain |
| Rpl30-ps9 | Ribosomal protein L30, pseudogene 9 | Unknown |
| Lrrc4b | Leucine rich repeat containing 4B | Regulates the formation of excitatory synapses |
| Mapt | Microtubule-associated protein tau | Promotes microtubule assembly and stability, and might be involved in the establishment and maintenance of neuronal polarity |
| Napg | N-ethylmaleimide sensitive fusion protein attachment protein gamma | Required for vesicular transport between the endoplasmic reticulum and the Golgi apparatus |
| Hnrnpa3 | Heterogeneous nuclear ribonucleoprotein A3 | Plays a role in cytoplasmic trafficking of RNA |
| Fos | FBJ osteosarcoma oncogene | Has a critical function in regulating the development of cells destined to form and maintain the skeleton. It is thought to have an important role in signal transduction and cell proliferation and differentiation |
DEGs, differentially expressed genes; METH, methamphetamine; FBJ, Finkel-Biskis-Jinkins; GTP, guanosine triphosphate.
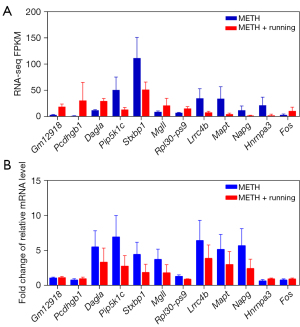
Validation of candidate genes related to running in METH-addicted mice
We used qRT-PCR to verify the authenticity of the results of RNA-seq. The most significant 12 genes were screened from the DEGs of exercise-METH addiction (Table 1), and qRT-PCR verified that the relative mRNA expression was consistent with the RNA-seq results (Figure 4B). Of the 8 genes, Gm12918, Pcdhgb1, and Fos were highly expressed, while Pip5k1c, Stxbp1, Lrrc4b, Mapt, and Napg were lowly expressed compared with the sedentary group. The candidate genes related to running exercise in the addicted mice could be used for further analysis.
GO and KEGG enrichment analysis
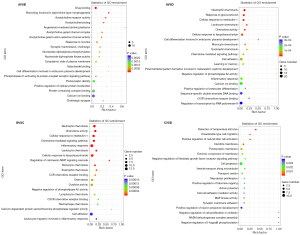
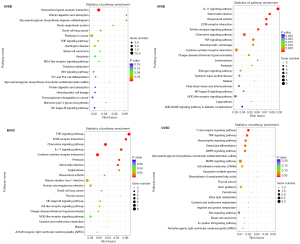
To explore the biological functions of DEGs, GO and KEGG enrichment analyses were conducted by comparing each of the two groups Saline and Saline + Running and METH and METH + Running (Figures 5,6). The enrichment of GO showed that the METH-addicted mice exhibited a drug binding function, affecting the branch involved in labyrinthine layer morphogenesis, whereas running exercise enriched neutrophil chemotaxis and glucocorticoid and cellular responses to interleukin-1 (IL-1) (Figure 5). These functions were found in both METH + Running versus METH and Saline + Running versus Saline groups. In addition, the KEGG enrichment analysis also showed running exercise impacted the function of the IL-17 signaling pathway, salmonella infection, and tumor necrosis factor (TNF) signaling pathway. These pathways were prominent and there were higher levels of factors in the METH + Running versus METH than in the other groups (METH + Running vs. Saline Running, Saline running vs. Saline, Saline vs. METH) (Figure 6). These results showed that running exercise plays a significant role in the immune responses of METH-addicted mice.
Discussion
The use of METH is a serious global public health problem with significant mental and medical consequences, including psychosis, dependency, overdose-death, and cognitive decline, as well as socio-economic and legal consequences (20). At present, drug therapy, cognitive behavioral therapy, and psychological therapy have achieved certain effects in the treatment of METH addicts. However, these therapies also have some prominent negative effects, including high drug development costs and adverse reactions, such as drug dependence. Additionally, no drug has yet been identified as an effective treatment for METH addiction. Therefore, it is necessary to find a more effective and lasting new treatment for METH addiction (3). The literature reveals that voluntary exercise can improve the level of brain-derived neurotrophic factor (BDNF) in the hippocampus, which has an important impact on learning and memory performance (21). On this basis, further effects of exercise (using free-running wheels) on brain plasticity may involve epigenetic modifications of the BDNF gene in the hippocampus, and these interactions may determine the ability of exercise to promote long-term changes in the brain to help cope with challenges (22). In addition, some researchers have also designed relevant exercise interventions for opioid addiction, such as long-term exercise or medium- and short-term swimming exercise, which can reduce the reward effect induced by morphine, thus reducing the pursuit of addictive drugs and reducing the risk of relapse after long-term withdrawal (23,24). Therefore, the effectiveness and applicability of physical exercise is feasible in drug rehabilitation programs. In summary, physical activity has been studied for some time as a combination therapy for other substance dependence, such as tobacco, alcohol, and marijuana use, with overall positive feedback for continued abstinence. In recent years, there have been clinical studies on METH. The METH users who have participated in physical exercise programs have shown better health indicators (as measured by significant improvements in aerobic capacity, muscle strength and endurance, body composition, and heart rate variability), reduced symptoms of depression and anxiety, lower relapse rates, and sustained abstinence compared to inactive individuals (20). Many studies have been conducted on exercise-based interventions for METH abuse management (25). Physical exercise reduces the extent, duration, and frequency of drug use in individuals with drug addiction during initiation of use, addiction after prolonged use, and during withdrawal and relapse (26). Moderate aerobic exercise, for example, is effective in reducing METH use by reducing depression and anxiety and controlling drug cravings in METH-addicted individuals (27-29). In addition, exercise intervention has had a positive effect on METH withdrawal (30,31). Similarly, it has been confirmed in animal studies that the self-administered METH dosage is significantly reduced in mice that voluntarily run on wheels (32-34). Since most drug users lead sedentary lives, studies have reported that rats were subjected to moderate endurance-forced exercise rather than voluntary exercise to mimic the exercise stress conditions found clinically (6,35). In this study, moderate-intensity forced running was used to intervene in the acute withdrawal period of METH-addicted mice. Behavioral experiments have shown that running could effectively alleviate the condition location bias of METH addiction and accelerate the recovery of METH addiction in mice. Previous studies have reported the effects of METH blocking exercise on the expression of Bdnf and Drd2 genes in the frontal cortex and striatum, but obviously there are few such reports (36). We screened DEGs by RNA-seq in the hippocampal region of the mouse brain, and the expression of DEGs was further verified by qRT-PCR. Running exercise could change the gene regulation in METH-addicted mice, with these genes closely related to the function of immune responses. There is no relevant report so far, and the experimental design is innovative at this stage. This can lay a preliminary foundation for further verifying the influence of DEGs on related pathways, and also provide reference for our team’s subsequent research. Clinical experiments have shown that, compared with those who did not exercise, METH users who exercised were able to improve their emotional state and general health status, enhance their cognitive function, reduce their recurrence rate, and sustain continuous abstinence, thus improving their overall quality of life (37). Exercise-based interventions or combination therapy are promising as useful tools for managing METH addiction (20). Long-term aerobic exercise has a good effect on METH addicts and can be used as an effective, drug-assisted therapy to help drug addicts overcome addiction (3).
Conclusions
Exercise could relieve the symptoms of addiction in METH-addicted mice. By analyzing RNA-seq transcriptome data on mice hippocampus, 12 DEGs significantly regulated by exercise were noted, which could be used for further research on regulation mechanisms. Exercise intervention is expected to be an effective treatment for relieving the symptoms of METH abuse in drug users.
Acknowledgments
The authors would like to thank all the team members. Without their support and patience, the experiment could not have been successfully completed.
Funding: This work was partly supported by the Central Public Welfare Research Institute (No. 81870458); the National Natural Science Foundation of China (No. 2019PT310003); the Fund for Yunling Scholar (No. YLXL20170002); the Yunnan Fundamental Research Projects (No. 202201AT070292); the Project for Innovation Team of Department of Science and Technology of Yunnan Province, China (No. 2018HC005); the Fund of Department of Education of Yunnan Province (No. 2019Y0352); the Fund of Health Commission of Yunnan Province (No. 2018NS0085); the Major Scientific and Technological Projects (biomedicine); the Biomedical Science Resource Database of the First Affiliated Hospital of Kunming Medical University (No. 202002AA100007); and the Yunnan Provincial Clinical Research Center for Skin Immune Diseases (No. 2019ZF012) at the Science and Technology Department of Yunnan Province.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-450/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-450/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-450/coif). All authors report that this work was supported partly from the Central Public Welfare Research Institute (No. 81870458); the National Natural Science Foundation of China (No. 2019PT310003); the Fund for Yunling Scholar (No. YLXL20170002); the Yunnan Fundamental Research Projects (No. 202201AT070292); the Project for Innovation Team of Department of Science and Technology of Yunnan Province, China (No. 2018HC005); the Fund of Department of Education of Yunnan Province (No. 2019Y0352); the Fund of Health Commission of Yunnan Province (No. 2018NS0085); Major Scientific and Technological Projects (biomedicine); the Biomedical Science Resource Database of the First Affiliated Hospital of Kunming Medical University (No. 202002AA100007); and the Yunnan Provincial Clinical Research Center for Skin Immune Diseases (No. 2019ZF012) at the Science and Technology Department of Yunnan Province. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Kunming Medical University and carried out in accordance with the relevant laws and regulations in China [ethics review approval No. kmmu20211261. Animal laboratory facility Permit Number: SYXK (Dian) K-2020-0006]. All the procedures were conducted according to the Chinese Guidelines for the Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction 2009;104:1085-99. [Crossref] [PubMed]
- Rawson RA. Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. J Food Drug Anal 2013;21:S77-81. [Crossref] [PubMed]
- Huang J, Zheng Y, Gao D, et al. Effects of Exercise on Depression, Anxiety, Cognitive Control, Craving, Physical Fitness and Quality of Life in Methamphetamine-Dependent Patients. Front Psychiatry 2019;10:999. [Crossref] [PubMed]
- Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol 2017;10:305-14. [PubMed]
- Zhou YU, Finlayson G, Liu X, et al. Effects of Acute Dance and Aerobic Exercise on Drug Craving and Food Reward in Women with Methamphetamine Dependence. Med Sci Sports Exerc 2021;53:2245-53. [Crossref] [PubMed]
- Jung S, Kim Y, Kim M, et al. Exercise Pills for Drug Addiction: Forced Moderate Endurance Exercise Inhibits Methamphetamine-Induced Hyperactivity through the Striatal Glutamatergic Signaling Pathway in Male Sprague Dawley Rats. Int J Mol Sci 2021;22:8203. [Crossref] [PubMed]
- Qi L, Yin Y, Bu L, et al. Acute VR competitive cycling exercise enhanced cortical activations and brain functional network efficiency in MA-dependent individuals. Neurosci Lett 2021;757:135969. [Crossref] [PubMed]
- Golsorkhdan SA, Boroujeni ME, Aliaghaei A, et al. Methamphetamine administration impairs behavior, memory and underlying signaling pathways in the hippocampus. Behav Brain Res 2020;379:112300. [Crossref] [PubMed]
- Keleta YB, Martinez JL. Brain Circuits of Methamphetamine Place Reinforcement Learning: The Role of the Hippocampus-VTA Loop. Brain Behav 2012;2:128-41. [Crossref] [PubMed]
- Pickens CL, Airavaara M, Theberge F, et al. Neurobiology of the incubation of drug craving. Trends Neurosci 2011;34:411-20. [Crossref] [PubMed]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther 2005;106:389-403. [Crossref] [PubMed]
- de Jong TV, Moshkin YM, Guryev V. Gene expression variability: the other dimension in transcriptome analysis. Physiol Genomics 2019;51:145-58. [Crossref] [PubMed]
- Zhao S, Fung-Leung WP, Bittner A, et al. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One 2014;9:e78644. [Crossref] [PubMed]
- Yoshizaki K, Asai M, Hara T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020;12:2036. [Crossref] [PubMed]
- Su HL, Zhu J, Chen YJ, et al. Roles of levo-tetrahydropalmatine in modulating methamphetamine reward behavior. Physiol Behav 2013;118:195-200. [Crossref] [PubMed]
- Qie X, Wen D, Guo H, et al. Endoplasmic Reticulum Stress Mediates Methamphetamine-Induced Blood-Brain Barrier Damage. Front Pharmacol 2017;8:639. [Crossref] [PubMed]
- Qu H, Liu R, Chen J, et al. Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway. Int J Environ Res Public Health 2020;17:2676. [Crossref]
- Ruilian L, Honglin Q, Jun X, et al. H2S-mediated aerobic exercise antagonizes the hippocampal inflammatory response in CUMS-depressed mice. J Affect Disord 2021;283:410-9. [Crossref] [PubMed]
- Fu K, Lin H, Miyamoto Y, et al. Pseudoginsenoside-F11 inhibits methamphetamine-induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens. Psychopharmacology (Berl) 2016;233:831-40. [Crossref] [PubMed]
- Morais APD, Pita IR, Fontes-Ribeiro CA, et al. The neurobiological mechanisms of physical exercise in methamphetamine addiction. CNS Neurosci Ther 2018;24:85-97. [Crossref] [PubMed]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004;20:2580-90. [Crossref] [PubMed]
- Gomez-Pinilla F, Zhuang Y, Feng J, et al. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci 2011;33:383-90. [Crossref] [PubMed]
- Rosa HZ, Barcelos RCS, Segat HJ, et al. Physical exercise modifies behavioral and molecular parameters related to opioid addiction regardless of training time. Eur Neuropsychopharmacol 2020;32:25-35. [Crossref] [PubMed]
- Carroll ME. Voluntary exercise as a treatment for incubated and expanded drug craving leading to relapse to addiction: Animal models. Pharmacol Biochem Behav 2021;208:173210. [Crossref] [PubMed]
- Brown RA, Abrantes AM, Read JP, et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act 2010;3:27-34. [Crossref] [PubMed]
- Lynch WJ, Peterson AB, Sanchez V, et al. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev 2013;37:1622-44. [Crossref] [PubMed]
- Haglund M, Ang A, Mooney L, et al. Predictors of depression outcomes among abstinent methamphetamine-dependent individuals exposed to an exercise intervention. Am J Addict 2015;24:246-51. [Crossref] [PubMed]
- R Rawson RA. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug Alcohol Depend 2015;156:21-8. [Crossref] [PubMed]
- Rawson RA, Chudzynski J, Gonzales R, et al. The Impact of Exercise On Depression and Anxiety Symptoms Among Abstinent Methamphetamine-Dependent Individuals in A Residential Treatment Setting. J Subst Abuse Treat 2015;57:36-40. [Crossref] [PubMed]
- Muller AE, Clausen T. Group exercise to improve quality of life among substance use disorder patients. Scand J Public Health 2015;43:146-52. [Crossref] [PubMed]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 2013;4:72. [Crossref] [PubMed]
- Miller ML, Vaillancourt BD, Wright MJ Jr, et al. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend 2012;121:90-6. [Crossref] [PubMed]
- Aarde SM, Miller ML, Creehan KM, et al. One day access to a running wheel reduces self-administration of D-methamphetamine, MDMA and methylone. Drug Alcohol Depend 2015;151:151-8. [Crossref] [PubMed]
- Engelmann AJ, Aparicio MB, Kim A, et al. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct 2014;219:657-72. [Crossref] [PubMed]
- Linke SE, Ussher M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse 2015;41:7-15. [Crossref] [PubMed]
- Thompson AB, Stolyarova A, Ying Z, et al. Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology 2015;99:658-64. [Crossref] [PubMed]
- Zhou Y, Zhao M, Zhou C, et al. Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front Neuroendocrinol 2016;40:24-41. [Crossref] [PubMed]
(English Language Editors: K. Gilbert and J. Jones)


