Preclinical systematic review & meta-analysis of cyclosporine for the treatment of myocardial ischemia-reperfusion injury
Introduction
Cyclosporine A (CsA) was first isolated in 1970 at Sandoz laboratories from a fungus found in a Norwegian soil sample (1). Sandoz had been trying to identify novel antibiotic compounds, but, in screening this compound, discovered that it had the ability to neutralize cytotoxic T cell activity in vitro (2). Subsequent in vivo studies further demonstrated its ability to suppress both antibody- and cell-mediated immunity (2). By the late 1970’s, CsA had been shown to promote graft survival in animal models of heart and kidney transplantation (3,4). This quickly led to clinical trials, which found similar benefits in human transplant recipients (5). This, combined with its low toxicity, led to CsA become the immunosuppressant drug of choice in the early days of solid organ transplantation and enabled the expansion of transplant programs worldwide.
The immunosuppressant effect of CsA is a result of calcineurin inhibition (6). Calcineurin is a phosphatase, whose activation of certain transcription factors leads to the upregulation of interleukin-2 and other cytokines important for initiating the T cell response. A secondary effect of CsA on mitochondria membrane permeability was later described by researchers trying to understand the mechanism behind CsA nephrotoxicity (7). It was discovered that CsA can bind to cyclophilin D, part of the mitochondria permeability transition pore (mPTP), preventing its opening during times of increased oxidative stress, which could otherwise lead to mitochondrial swelling, disruption of the electron transport chain, and eventual rupture (8).
Researchers soon realized that this property of CsA could mitigate ischemia-reperfusion injury (IRI) caused by transient loss of blood flow to an organ or tissue. This was demonstrated in animal models involving various organs, including the heart and kidney (9,10). There was particular interest in using CsA to protect the myocardium from IRI following revascularization, such as after coronary artery thrombosis.
Despite promising studies in animals, attempts to translate findings into the clinical realm produced mixed results. An initial pilot randomized controlled trial (RCT) of 58 patients conducted across several hospital in France found that CsA given at 2.5 mg/kg at the onset of reperfusion in patients undergoing percutaneous coronary intervention (PCI) led to smaller infarct size and decreased creatinine kinase (CK) levels (11). However, the subsequent larger trial involving 970 patients failed to show any clinical benefit and found that CsA did not reduce the risk of adverse left ventricular remodeling at 1 year (12).
In order to better understand the failure of CsA to translate clinically, it is worthwhile to return to the preclinical studies that informed clinical trials. The purpose of this review is to summarize evidence in the preclinical literature for the benefit of CsA in IRI. In addition to elucidating possible reasons for the failure of preclinical studies to translate into the clinical realm, we sought to identify gaps that should be addressed before moving forward with any further clinical studies aiming to use CsA to mitigate IRI in the heart or other organs. Our search included models of IRI in any organ; however, in this article we will summarize only cardiac studies to allow for a more in-depth analysis. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-618/rc).
Methods
Database & literature search strategies
The proposed systematic review was prospectively registered in the online international registry PROSPERO (www.crd.york.ac.uk/prospero/) with the unique ID CRD42020159620. Searches were conducted on March 9, 2022 by a health librarian/expert searcher (SC) of the health research databases Ovid MEDLINE, Ovid EMBASE, Web of Science BIOSIS, and Scopus, as well as the Cochrane Database of Systematic Reviews and the PROSPERO database of systematic review protocols. Keywords and controlled vocabulary (e.g., MeSH, EMTREE) were used to identify studies related to the concepts: “reperfusion injuries” and “cyclosporin”. In vitro studies were excluded. No other limits were applied. Searches were adjusted appropriately for each database. Results were exported to Covidence review management software (www.covidence.org) and duplicates were automatically removed. A detailed search strategy is included in the Appendix 1.
Eligibility criteria
The primary aim of the review was to include all non-human in vivo animal studies of IRI, using CsA as a treatment. There was no exclusion of studies based on species, language, date of publication or type of publication (e.g., paper, brief communication, abstract). Non-experimental publications, as well as in vitro and ex vivo (i.e., isolated perfused organs) studies were excluded. Studies were excluded if they did not have an appropriate control group for comparison (i.e., ischemia-reperfusion alone) or if CsA was used for another indication (e.g., at high doses to cause nephrotoxicity). Studies not reporting clinically relevant outcomes were also excluded. Clinically relevant outcomes were taken as routine serum biochemistry (e.g., troponin, creatinine, lactate, liver transaminases), infarct size, histological assessment of injury, organ function, and survival. Human studies were excluded last, with the intention for them to be analyzed separately if appropriate. Different publications presenting identical data (e.g., conference abstracts and full-length papers by the same authors) were excluded, however, publications presenting non-identical data from the same authors were included to minimize the risk of publication bias.
Studies were reviewed in two stages. First, a title and abstract review was conducted independently by two reviewers (JH and BAMG). This was followed by a full text review, applying the same inclusion/exclusion criteria. Conflicts were resolved by means of consensus between the two reviewers.
Data extraction
Data was retrieved from selected studies by a single reviewer (JH). Data extracted included animal characteristics (species, strain, sex, age, number per group), experimental characteristics (dose of CsA, timing of drug administration, duration of ischemia, blood vessel occluded), and animal outcomes (infarct size, biochemical markers of injury, histological evidence of injury, markers of organ function, survival). Data was extracted manually from graphs if it was not listed explicitly.
Quality assessment
Bias was assessed using the Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE) risk of bias tool, as well as a modified Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist (13,14). Though any type of publication was included in our review, only full-length articles were assessed for bias, as this was impractical for conference abstracts and brief reports due to their lack of detail.
Statistical analysis
Meta-analysis was conducted using RevMan 5 software (Cochrane). Only coronary occlusion studies reporting infarct size were included, as this was the most common study design and most common reported outcome. Results were reported as weighted mean differences since all studies used the same unit measure (percentage of area at risk). Results from abstracts were included in the analysis only if there were no subsequent full-length publications of the same study (to avoid duplication of results). Different treatment groups within the same study were treated separately. A random effects model was chosen due to the statistical and methodological heterogeneity of the studies. Subgroup meta-analyses were planned based on age, sex, species, dose, timing of administration, and ischemia duration, if appropriate.
Results
Study inclusion
The PRISMA diagram for the systematic review is presented in Figure 1. Our initial search yielded 2,070 unique records. At the abstract review phase, the kappa score between reviewers was 0.98, indicating almost perfect agreement. After abstract screening, 625 studies remained for full text review, 164 of which were ultimately included as preclinical studies. The full text of 67 studies could not be found despite extensive searching through online databases and physical records. The majority of these were either conference abstracts (37/67) or from smaller, non-English language journals (12/67). The numbers of included studies were further broken down by organ. Given the high number of records identified, this article will deal with only cardiac IRI, which includes 71 total studies (see Appendix 1 for full list of included articles).

Risk of bias assessment
Risk of bias was assessed using SYRCLE’s risk of bias tool, as well as a modified CAMARADES checklist. Using SYRCLE’s tool, the majority of categories had either high or unclear risk of bias across studies (Figure 2A). The risk of bias being unclear was mainly due to studies lacking sufficient detail about procedures, such as randomization, allocation concealment, and handling of baseline characteristics. We found low risk of bias related to selective outcome reporting. Though no study had a prespecified protocol available, we did find that the majority of studies were consistent in reporting all outcomes described in the methods. We also found that studies were largely free of other important sources of bias, such as contamination, unit analysis error or the inappropriate influence of funders. Results from the CAMARADES checklist (Figure 2B) additionally highlighted other potential areas of bias, such as lack of sample size calculation, unclear conflicts of interest, and confirmation of ischemia.

Study characteristics
Of the 71 cardiac studies identified, 13 (18%) were conference abstracts, while 58 (82%) were full-length articles. The majority of cardiac studies (59/71, 83%) used a model of coronary artery occlusion, most commonly occlusion of the left anterior descending artery (36/59, 61%) (Appendix 1). Other models included cardiac arrest, cardiopulmonary bypass (CPB), hypoxia, and one study of CsA for the treatment of IRI in cardiac transplantation (Appendix 1).
Studies employed a variety of animals, including mice, rats, rabbits, pigs, and sheep. However, rats were the most common animal, used in 42% of studies (30/71). CsA was most commonly administered as a single dose, intravenously or intraperitoneally, though several studies either pretreated the animal with CsA for several days prior or continued dosing CsA up to 24 hours post-ischemia. Doses ranged from 0.25 to 40 mg/kg, with 10 mg/kg being the most common, used in 51% (36/71) of studies. Regarding studies of myocardial IRI through coronary artery occlusion, the most common duration of ischemia was 30 minutes [59% (35/59) of studies], though ischemic times ranged from 5 to 90 minutes. The majority of studies administered CsA during the period of myocardial ischemia [58% (34/59)]. Fewer studies administered CsA prior to myocardial ischemia [22% (13/59)] or following reperfusion [i.e., post-ischemia; 12% (7/59)], while a minority administered CsA both before and after myocardial ischemia (multiple doses) or during and after myocardial ischemia (multiple doses or continuous infusion).
Study outcomes
Overall, 75% (53/71) of the studies reported a clear positive effect of CsA in mitigating myocardial IRI by some clinically relevant parameter, such as infarct size, serum troponin, or cardiac function parameters (e.g., cardiac output, cardiac index). However, some studies testing multiple doses reported no positive effect with the lowest tested dose. Coronary artery occlusion studies most commonly reported infarct size [reported in 93% (55/59) of studies], given as the percentage of the myocardium at risk. Of these, 80% (44/55) reported a reduction in infarct size with CsA. Serum troponin and/or CK or cardiac function parameters (e.g., cardiac output or cardiac index) were less commonly reported with coronary occlusion studies. Only 2 of the 4 studies testing CsA in cardiac arrest showed positive effects on cardiac parameters following resuscitation and only 2 of the studies testing CsA in CPB reported post-CPB cardiac output, with no observed benefit. Three studies of anoxia in a piglet model reported a positive effect of CsA in post-hypoxia cardiac function and troponin, though they were all published by the same group. A minority of studies (4%, 3/71) reported histologic findings exclusively.
Meta-analysis of coronary artery occlusion studies
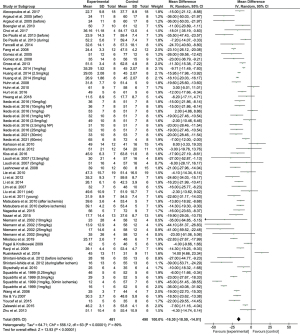
Combining the results of all suitable coronary artery occlusion studies (43/59), we found an overall positive effect of CsA administration in reducing infarct size, with a combined reduction of 16.09% (95% CI: 13.67% to 18.50%) of infarct size as a percentage of the area at risk (Figure 3). Statistical heterogeneity between studies, however, was found to be high (I2=89%), suggesting this effect may be due to study differences rather than a true effect of the treatment. This is similarly reflected in the funnel plot (Figure 4), whose asymmetry may be explained, in part, by statistical heterogeneity.

Subgroup analysis was undertaken to uncover the potential effects of various study differences. We performed meta-analyses grouping studies by species, sex, age, dose, and ischemia time. A similar overall effect was seen between mouse, rat, and rabbit studies (Appendix 1). However, the effect of CsA became non-significant (P=0.08) when considering only porcine studies (Figure 5A). The five porcine studies also had lower statistical heterogeneity (I2=48%) compared to the other species subgroups. Similarly, the effect of CsA disappeared when considering studies that included only female animals (P=0.88), though this subgroup included only three studies (Appendix 1). Combining studies including older animals (rodents 20–24 months) likewise showed a non-significant effect (P=0.14), though this was not statistically different from the combined effect seen in studies containing young animals (P=0.48) (Figure 5B).

Studies administering CsA prior to ischemia showed a greater reduction in infarct size (22.86%; 95% CI: 17.73% to 27.98%) compared to those administering CsA during or after ischemia (Appendix 1). The test for subgroup differences was statistically significant (P=0.01). A subgroup meta-analysis of studies by dose likewise showed a greater reduction in infarct size with doses ≥12.5 mg/kg (22.36%; 95% CI: 17.26% to 27.46%), however this effect was not statistically different from other subgroups (P=0.09) (Appendix 1). The overall effect of CsA on infarct size reduction was lower in studies with ischemic times greater than 40 minutes (8.63%; 95% CI: 4.25% to 13.01%) compared to other studies (P=0.002), but still remained positive (P=0.0001) (Figure 5C, Appendix 1). Despite differences observed between subgroups, heterogeneity within most subgroups remained high (I2>70%).
Discussion
In this systematic review of preclinical studies administering CsA to mitigate myocardial IRI, we found that the majority of studies reported a clearly positive effect on various clinically relevant parameters. A meta-analysis of 43 studies utilizing coronary artery occlusion demonstrated an overall reduction in infarct size with the use of CsA (Figure 3). In stark contrast, several clinical studies have been conducted with weak or non-effective benefit (12,15). Understanding this discrepancy between positive results in small and large animals and negative results in clinical practice is vital. Importantly, subgroup meta-analyses suggest that the effect of CsA may differ based on species, sex, age, timing of administration, and ischemia duration.
The findings of these multiple studies contrast with clinical trials, which have shown mixed results at best. The largest trial, published by Cung et al. (12) in 2015, included 970 patients with presenting with anterior ST-elevation myocardial infarction (STEMI) undergoing PCI randomized to receive 2.5 mg/kg of CsA or placebo immediately prior to reperfusion. They found that CsA conferred no benefit on multiple clinical parameters, including death, worsening heart failure, and left ventricular remodeling. The concurrently run trial (using the same dose), published by Ottani et al. (15) in 2016, which included 410 STEMI patients undergoing PCI, similarly found no difference in multiple cardiac-specific outcomes, including ST-segment resolution, serum troponin, and left ventricular ejection fraction.
There are notable differences between these trials and the preclinical studies identified by our search, such as animal age, health, CsA dose, duration of ischemia, and timing of dose, as well as species differences (i.e., humans versus research animals) that could potentially explain the discrepancies in outcomes. Animal age was not commonly reported for rabbits or pigs, but most rodent studies used animals between 8 and 12 weeks old, which is roughly equivalent to a young adult or even adolescent human (16). In both of the large RCTs testing CsA in reperfusion following STEMI, the average age of patients was close to 60 (12,15). Only three studies were identified that used older animals (rodents aged 20–24 months), two of which showed no effect of CsA on infarct size (17-19). While Cung et al. (12) did include a subgroup analysis of patients older and younger than 75, which showed no difference in clinical outcomes, patients in the younger group were still quite a bit older, relative to the animals used in preclinical studies. Furthermore, the animals used in these studies were typically disease free. Only one study was identified that tested the ability of CsA to reduce infarct size in a co-morbid animal (pre-diabetic, obese Zucker rats) and found no effect on infarct size (20). In contrast, participants in the clinical trials by Cung et al. and Ottani et al. were often co-morbid, with type 2 diabetes and hypertension being common, as well as being overweight (12,15). While young, healthy animals may be appropriate for initial investigations, moving toward an animal model that is more representative of the clinical population to which the intervention would likely be applied should be considered prior to proceeding with costly clinical trials.
Our meta-analysis of subgroups divided by species suggested that CsA could be less effective in pigs (Figure 4). This is somewhat confounded by the fact that all of the pig studies used mixed sex or female only animals. However, it does highlight the importance of considering species differences when interpreting preclinical studies. For pharmacological interventions, particular attention should be paid to the specific pathways of metabolism for the drug of interest. CsA is metabolised by the cytochrome P450-3A family of enzymes (21). Not only does the kinetic activity of cytochrome P450 (CYP450) enzymes differ between animals and humans, it appears that there is no one animal whose CYP450 enzymatic activity best matches that of humans across multiple metabolites (22). This does not even take into consideration differences between individuals, which is likely more pronounced in human populations than the inbred animal strains used for most biomedical research. Seeing a consistent effect across a variety of species and strains increases confidence that the intervention will work in human studies.
Grouping studies by duration of ischemia, we found that the effect of CsA in reducing infarct size was significantly reduced for ischemic times longer than 40 minutes. Average ischemic times in the studies by Cung et al. and Ottani et al. were 4.5 and 3 hours, respectively (12,15). In both studies, more than 80% of patients had no flow through the occluded vessel [i.e., thrombolysis in myocardial infarction (TIMI) score of 0], as was the case in all but one of the animal studies identified. In clinical practice, it is rare to have ischemia of such short duration in acute coronary thrombosis, given the time that is taken for patients to present, diagnosis to occur, and treatment to be initiated. Though a target of 90 minutes from presentation to PCI is recommended by the American Heart Association, shorter time to reperfusion (e.g., less than 60 minutes) has been shown to be associated with decreased mortality (23). Similarly, we did find a significant difference between subgroups divided by timing of administration, with dosing prior to ischemia being more effective at reducing infarct size. This is relevant, as it would be impossible to administer CsA prior to unexpected ischemia as occurs in the setting of myocardial infarction (MI), but CsA could be given prior to known periods of ischemia, such as during cardiac surgery or transplantation.
It is worth noting that our subgroup meta-analysis did not suggest an effect based on dose. All clinical trials of CsA have used doses of 2.5 mg/kg, while preclinical studies tended to use higher doses (with 10 mg/kg being most common). The overall effect from studies using doses of 12.5 mg/kg or more showed greater reduction in infarct size, however, this was not significantly different from other subgroups. This was true even after eliminating studies using nanoparticle formulations, which tended to show greater benefit with lower doses (24,25). It may be that for this particular drug the effect on mitochondria is not gradational, but rather exhibits more of a threshold effect, below which it is ineffective (or at least a very narrow range in which increased doses will result in increased effect).
As alluded to previously, the goal of this systematic review is largely hypothesis generating. The suggestions gleaned from meta-analyses of subgroups should be understood within certain limitations. An important caveat for interpreting the results of the meta-analyses is the high degree of statistical heterogeneity observed between studies, which remained largely unchanged despite grouping studies according to several different methodological considerations. It does not appear that the heterogeneity can be entirely explained by dose, timing of administration, duration of ischemia or species (though the heterogeneity for porcine studies was low). It may be a result of a combination of these factors. Methodology is another consideration to explain heterogeneity in results, especially given the high degree of variation in their results compared to others. The majority of studies purported to be measuring infarct size by injecting Evans blue dye, which is a well-established technique, though may lead to variability in unskilled hands. Particularly with preclinical studies, there is always the concern for publication bias, which can contribute to heterogeneity. As well, selective reporting of results (i.e., omitting negative results) could also be a factor and is not easily detectable in preclinical studies.
Another important limitation is the high risk for bias seen in these studies. Animal studies are typically far less diligent in following standard practices that are commonly used to minimize bias in clinical trials (e.g., randomization, allocation concealment, blinding during analysis) (26). They are also less detailed in their description of methods taken to minimize bias. For instance, while several studies indicated that they randomized animals, they did not include sufficient detail to judge whether this was properly done (e.g., using a random number table or generator, as opposed to assigning every other animal to a group). It is important to encourage the implementation of these bias-reducing methods in preclinical studies, as this will, not only increase confidence in study results, but reduce the chance of obtaining false positive results.
We would like to acknowledge a systematic review posing a similar question, published by Lim et al. in 2012 (27). They similarly found an overall positive effect, while commenting on several discrepancies, such as between species. In addition to updating and broadening the search results, which resulted in the addition of 23 studies for meta-analysis, we have added extended subgroup meta-analyses. As well, we now have the opportunity to interpret the findings of our systematic review and meta-analysis in light of the data from several large clinical trials.
Overall, our systematic review identified multiple preclinical studies that tested CsA for the treatment of myocardial IRI. Their indication of an overwhelmingly positive effect is in contrast with the results from clinical studies. Our meta-analysis identified several factors that potentially contributed to these discrepancies. It may not be worthwhile to further explore these in animal studies of myocardial ischemia, given that the clinical trials have already been conducted. However, our findings highlight the potential pitfalls of translating the results of preclinical studies that should be considered prior to initiating clinical trials.
Acknowledgments
Thank you to the support of our fellow members of the Freed and Shapiro labs at the University of Alberta.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-618/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-618/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-618/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Colombo D, Ammirati E. Cyclosporine in transplantation–- a history of converging timelines. J Biol Regul Homeost Agents 2011;25:493-504. [PubMed]
- Borel JF, Kis ZL, Beveridge T. The History of the Discovery and Development of Cyclosporine (Sandimmune®). In: Merluzzi VJ, Adams J. editors. The Search for Anti-Inflammatory Drugs: Case Histories from Concept to Clinic. Boston, MA, USA: Birkhäuser, 1995:27-63.
- Homan WP, French ME, Millard P, et al. Studies on the effects of cyclosporin A upon renal allograft rejection in the dog. Surgery 1980;88:168-73. [PubMed]
- Kawahara K, Sutherland DE, Rynasiewicz JJ, et al. Prolongation of heterotopic cardiac allografts in rats by cyclosporin A. Surgery 1980;88:594-600. [PubMed]
- Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979;2:1033-6. [Crossref] [PubMed]
- Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without... J Immunol 2013;191:5785-91. [Crossref] [PubMed]
- Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 1987;19:297-303. [Crossref] [PubMed]
- Tanveer A, Virji S, Andreeva L, et al. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. Eur J Biochem 1996;238:166-72. [Crossref] [PubMed]
- Kurokawa T, Kobayashi H, Nonami T, et al. Beneficial effects of cyclosporine on postischemic liver injury in rats. Transplantation 1992;53:308-11. [Crossref] [PubMed]
- Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol 1991;23:1351-4. [Crossref] [PubMed]
- Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473-81. [Crossref] [PubMed]
- Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med 2015;373:1021-31. [Crossref] [PubMed]
- Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCL’'s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [Crossref] [PubMed]
- Sayour AA, Celeng C, Oláh A, et al. Sodium-glucose cotransporter 2 inhibitors reduce myocardial infarct size in preclinical animal models of myocardial ischaemia-reperfusion injury: a meta-analysis. Diabetologia 2021;64:737-48. [Crossref] [PubMed]
- Ottani F, Latini R, Staszewsky L, et al. Cyclosporine A in Reperfused Myocardial Infarction: The Multicenter, Controlled, Open-Label CYCLE Trial. J Am Coll Cardiol 2016;67:365-74. [Crossref] [PubMed]
- Wang S, Lai X, Deng Y, et al. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci 2020;242:117242. [Crossref] [PubMed]
- Liu L, Zhu J, Brink PR, et al. Age-associated differences in the inhibition of mitochondrial permeability transition pore opening by cyclosporine A. Acta Anaesthesiol Scand 2011;55:622-30. [Crossref] [PubMed]
- Youcef G, Belaidi E, Waeckel L, et al. Tissue kallikrein is required for the cardioprotective effect of cyclosporin A in myocardial ischemia in the mouse. Biochem Pharmacol 2015;94:22-9. [Crossref] [PubMed]
- Zhu J, Rebecchi MJ, Wang Q, et al. Chronic Tempol treatment restores pharmacological preconditioning in the senescent rat heart. Am J Physiol Heart Circ Physiol 2013;304:H649-59. [Crossref] [PubMed]
- Huhn R, Heinen A, Hollmann MW, et al. Cyclosporine A administered during reperfusion fails to restore cardioprotection in prediabetic Zucker obese rats in vivo. Nutr Metab Cardiovasc Dis 2010;20:706-12. [Crossref] [PubMed]
- Christians U, Sewing KF. Cyclosporin metabolism in transplant patients. Pharmacol Ther 1993;57:291-345. [Crossref] [PubMed]
- Bogaards JJ, Bertrand M, Jackson P, et al. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica 2000;30:1131-52. [Crossref] [PubMed]
- Park J, Choi KH, Lee JM, et al. Prognostic Implications of Door-to-Balloon Time and Onset-to-Door Time on Mortality in Patients With ST -Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Heart Assoc 2019;8:e012188. [Crossref] [PubMed]
- Ikeda G, Matoba T, Ishikita A, et al. Nanoparticle-Mediated Simultaneous Targeting of Mitochondrial Injury and Inflammation Attenuates Myocardial Ischemia-Reperfusion Injury. J Am Heart Assoc 2021;10:e019521. [Crossref] [PubMed]
- Ikeda G, Matoba T, Nakano Y, et al. Nanoparticle-Mediated Targeting of Cyclosporine A Enhances Cardioprotection Against Ischemia-Reperfusion Injury Through Inhibition of Mitochondrial Permeability Transition Pore Opening. Sci Rep 2016;6:20467. [Crossref] [PubMed]
- Macleod MR, Lawson McLean A, Kyriakopoulou A, et al. Risk of Bias in Reports of In Vivo Research: A Focus for Improvement. pLoS Biol 2015;13:e1002273. [Crossref] [PubMed]
- Lim WY, Messow CM, Berry C. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: a systematic review and meta-analysis. Br J Pharmacol 2012;165:2034-43. [Crossref] [PubMed]