
Early administration of dobutamine in the treatment of septic shock patients with tumor-a retrospective comparative cohort study
Introduction
The concept of sepsis has been revised to an uncontrolled body response to infection leading to life-threatening organ dysfunction (1). For a long time, infection, systemic inflammatory response syndrome, sepsis, septic shock, and multiple organ dysfunction syndrome have been thought to be related to the different stages of the development of the same important pathophysiological process (2,3).
Septic cardiomyopathy is proposed in the guidelines for rescuing sepsis (4,5). For adult patients with septic shock, the use of a first-line vasopressor is recommended to administer norepinephrine. When accompanied by cardiac insufficiency, circulatory perfusion is unable to ensure sufficient volume and arterial blood pressure. It is recommended that dobutamine combined with norepinephrine or epinephrine alone be used. Fink et al. showed that dobutamine can improve liver perfusion after experimental shock (6). In comparing septic rats with or without dobutamine pretreatment, they found that dobutamine pretreatment can activate β1 adrenergic receptors and improve the survival rate, liver microcirculation, and liver function after sepsis. Similarly, dobutamine can protect renal function in patients with septic shock, improve renal perfusion, increase the glomerular filtration rate, and increase serum creatinine without significantly increasing urine volume and the fractional sodium excretion clearance rate. However, the timing of dobutamine use is not clear.
Additionally, a study by Mirouse et al., which aimed to explore the similarities and differences between tumors and sepsis, found that the 2 diseases have many related pathophysiological properties due to the inability of the body’s immune system to respond to injury (the former is caused by malignant cells, the latter is caused by the invasion of pathogens into the body), and the series of changes in the immune homeostasis of the 2 diseases may affect each other (7). In a previous study, Dimopoulos et al. found that a history of stage I or II solid tumors was an independent risk factor for 28-day mortality in sepsis (8). Further, Kim et al. showed that the 30-day and 1-year mortality rates were 52.1% and 81.3% in adult sepsis shock patients with cancer (9). There is also a lack of relevant research on the effect of dobutamine on septic shock patients with tumor.
The present study explored the therapeutic effect of dobutamine in the treatment of septic shock patients with tumors. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3226/rc).
Methods
Subjects
This was a retrospective comparative cohort study. This study retrospectively analyzed 122 septic shock patients with tumors at the Sun Yat-sen University Cancer Center from June 2008 to November 2021. Patients who were diagnosed with cancer, met the diagnostic criteria for septic shock (as per the sepsis 3.0 guidelines) and who received anti-tumor medical treatment were included in the study. Patients who suffered from previous severe heart failure, and severe liver and kidney insufficiency in combination with other autoimmune diseases were excluded from the study, as were those who died from acute coronary syndrome, epilepsy, acute cerebral infarction or cerebral hemorrhage and those who had rapid tumor progression due to the poor control of the primary tumor. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. SL-B2021-410-01). Individual consent for this retrospective analysis was waived.
Data collection
In this study, basic information about patients, their tumor stage, treatment plan, vital signs during hospitalization, various tests, examination results, the highest Sequential Organ Failure Assessment (SOFA) score, the highest Acute Physiology and Chronic Health Evaluation II (APACHE-II) score within 24 hours of admission to the intensive care unit (ICU), ICU admission duration, and the duration of mechanical ventilation was collected.
Grouping and treatment
All patients were screened according to the septic shock guidelines, and patients were allocated to the following groups: (I) the early administration group (<3 days), who received dobutamine within 3 days from the start of septic shock; (II) the late administration group (≥3 days), who received dobutamine ≥3 days after the start of septic shock; and (III) the non-administration group, who did not receive dobutamine in the treatment process for septic shock.
The drug manufacturer of dobutamine in this study was Shanghai Shangyao No.1 Biochemical Pharmaceutical Co., Ltd. The specification was 2 mL:20 mg. As recommended, 5% glucose solution or 0.9% sodium chloride injection was added to the dobutamine after dilution for micro-pumping. The dose range in this study was 0.62–4.68 µg/kg/min, and the average continuous pumping time was 5 days.
Statistical analysis
This study used the statistical analysis software SPSS (version 26.0) and R (version 4.0.2). We compared the measurement data using the Student’s t-test, and the count data using the χ2 test (or Fisher exact test as indicated). The survival curves were plotted using the Kaplan-Meier method, and the 28-day mortality differences were compared among the different groups of patients using log-rank tests. Additionally, the Cox proportional hazards regression model was used to calculate the hazard ratios (HRs) and 95%confidence intervals (CIs). P<0.05 indicated that the difference was statistically significant.
Results
Baseline characteristics of the 122 sepsis shock patients with tumors
A total of 122 tumor patients were included in this study, including the early administration group (<3 days, n=15), the late administration group (≥3 days, n=22), and the non-administration group (n=85). The clinical data are shown in detail in Table 1. Among them, there were 86 male patients and 36 female patients, with a male to female ratio of 2.4:1 (P=0.333). The age of the patients in the total population was 53.11±17.25 years old, and there was no significant difference in age among the 3 groups (P=0.169). In the total population, there were 82 patients with no underlying medical history, 0 patients with coronary heart disease, 18 patients with hypertension, 5 patients with diabetes, and 17 patients with viral hepatitis B (P=0.688). In the total population, there were 54 patients with lymphohematopoietic tumors and 68 patients with solid tumors, with a ratio of 1.26:1 (P=0.373). Among the patients with solid tumors, there were 22 cases (32.35%) of nasopharyngeal carcinoma, 17 cases (25%) of lung cancer, 14 cases (20.59%) of digestive system tumors, and 15 cases (22.06%) of other tumors. Among the cases, 1 (1.47%) was in stage 0, 0 were in stage I, 4 were in stage II (5.88%), 15 were in stage III (22.06%), and 48 were in stage IV (70.59%). The clinical stages of the three groups were not statistically significant (P=0.396).
Table 1
| Characteristics | The early group (N=15) | The late group (N=22) | The non-use group (N=85) | P |
|---|---|---|---|---|
| Age, years (mean ± SD) | 57.87±10.89 | 57.36±14.10 | 51.16±18.61 | 0.169 |
| Gender, n (%) | 0.333 | |||
| Female | 6 (40.0) | 4 (18.2) | 26 (30.6) | |
| Male | 9 (60.0) | 18 (81.8) | 59 (69.4) | |
| Height, cm (mean ± SD) | 162.13±7.14 | 165.06±5.80 | 164.64±9.85 | 0.568 |
| Weight, kg (mean ± SD) | 59.02±8.67 | 56.79±10.49 | 56.81±12.01 | 0.781 |
| Underlying diseases, n (%) | 0.688 | |||
| None | 7 (46.7) | 16 (72.7) | 59 (69.4) | |
| Coronary heart disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertensive | 3 (20.0) | 3 (13.6) | 12 (14.1) | |
| Diabetes mellitus | 1 (6.7) | 1 (4.5) | 3 (3.5) | |
| Viral hepatitis B | 4 (26.7) | 2 (9.1) | 11 (12.9) | |
| Diagnosis, n (%) | 0.373 | |||
| Hematological tumor | 8 (53.3) | 7 (31.8) | 39 (45.9) | |
| Solid tumor | 7 (46.7) | 15 (68.2) | 46 (54.1) | |
| Nasopharyngeal carcinoma | 2 (28.6) | 4 (26.7) | 16 (34.8) | |
| Lung cancer | 3 (42.9) | 6 (40.0) | 8 (17.4) | |
| Gastroenteric carcinoma | 0 (0.0) | 1 (6.7) | 13 (28.3) | |
| Others | 2 (28.6) | 4 (26.7) | 9 (19.6) | |
| Solid tumor stage, n (%) | 0.396 | |||
| Stage 0 | 0 (0.0) | 0 (0.0) | 1 (2.2) | |
| Stage I | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Stage II | 1 (14.3) | 0 (0.0) | 3 (6.5) | |
| Stage III | 0 (0.0) | 2 (13.3) | 13 (28.3) | |
| Stage IV | 6 (85.7) | 13 (86.7) | 29 (63.0) | |
| Tumor treatment, n (%) | 0.125 | |||
| Multimodality therapy | 4 (26.7) | 11 (50.0) | 40 (47.1) | |
| Chemotherapy | 11 (73.3) | 10 (45.5) | 45 (52.9) | |
| Targeted therapy | 0 (0.0) | 1 (4.5) | 0 (0.0) | |
| Myelosuppression grade, n (%) | 0.565 | |||
| Grade 0 | 0 (0.0) | 1 (4.5) | 0 (0.0) | |
| Grade I | 0 (0.0) | 0 (0.0) | 2 (2.4) | |
| Grade II | 2 (13.3) | 2 (9.1) | 7 (8.2) | |
| Grade III | 2 (13.3) | 3 (13.6) | 7 (8.2) | |
| Grade IV | 11 (73.3) | 16 (72.7) | 69 (81.2) | |
| Transfusion of blood products, n (%) | 0.581 | |||
| No | 4 (26.7) | 3 (13.6) | 19 (22.4) | |
| Yes | 11 (73.3) | 19 (86.4) | 66 (77.6) | |
| GM-CSF, n (%) | 0.165 | |||
| No | 4 (26.7) | 9 (40.9) | 18 (21.2) | |
| Yes | 11 (73.3) | 13 (59.1) | 67 (78.8) | |
| Site of infection, n (%) | 0.176 | |||
| Lung | 15 (100.0) | 22 (100.0) | 72 (84.7) | |
| Abdominal | 0 (0.0) | 0 (0.0) | 10 (11.8) | |
| Bloodstream | 0 (0.0) | 0 (0.0) | 3 (3.5) | |
| Culture of humoral specimen, n (%) | 0.128 | |||
| Gram-positive bacteria | 1 (6.7) | 5 (22.7) | 4 (4.7) | |
| Gram-negative bacteria | 8 (53.3) | 8 (36.4) | 33 (38.8) | |
| Fungi | 0 (0.0) | 1 (4.5) | 2 (2.4) | |
| Undefined | 6 (40.0) | 8 (36.4) | 46 (54.1) | |
| Procalcitonin, ng/mL (mean ± SD) | 13.18±14.44 | 23.23±33.17 | 35.43±54.45 | 0.192 |
| Vital signs (mean ± SD) | ||||
| Heart rate, bpm | 137.33±20.41 | 139.68±18.42 | 129.76±41.22 | 0.447 |
| Respiratory rate, bpm | 25.33±4.84 | 28.27±5.63 | 27.06±9.52 | 0.588 |
| Systolic pressure, mmHg | 88.47±19.04 | 89.27±15.19 | 79.34±23.16 | 0.08 |
| Diastolic pressure, mmHg | 54.07±10.59 | 54.50±11.79 | 54.33±9.30 | 0.992 |
| Mean arterial pressure, mmHg | 65.53±12.23 | 66.09±12.38 | 64.12±9.07 | 0.673 |
| The blood routine (mean ± SD) | ||||
| WBC, ×109/L | 6.14±6.64 | 14.60±20.91 | 9.17±11.35 | 0.122 |
| RBC, ×1012/L | 2.92±0.91 | 3.06±1.11 | 2.89±0.77 | 0.717 |
| HGB, g/L | 91.86±29.19 | 89.76±25.06 | 86.65±21.41 | 0.661 |
| PLT, ×109/L | 60.27±63.47 | 102.68±100.98 | 76.01±94.37 | 0.345 |
| The biochemical test (mean ± SD) | ||||
| ALT, U/L | 35.95±29.27 | 124.39±293.94 | 229.73±708.72 | 0.457 |
| Albumin, g/L | 29.67±5.06 | 28.24±4.72 | 28.16±4.94 | 0.547 |
| TBIL, umol/L | 24.68±21.44 | 37.69±64.15 | 35.45±44.69 | 0.672 |
| Creatinine, umol/L | 114.43±63.95 | 109.52±80.47 | 114.84±87.33 | 0.965 |
| CRP, mg/L | 140.68±116.58 | 169.08±110.43 | 195.73±114.04 | 0.182 |
| Arterial blood gas analysis (mean ± SD) | ||||
| pH | 7.34±0.18 | 7.30±0.17 | 7.35±0.13 | 0.241 |
| Oxygenation index | 175.86±113.19 | 148.71±100.48 | 172.80±101.68 | 0.597 |
| Lactate, mmol/L | 5.27±3.18 | 5.02±4.90 | 5.38±5.45 | 0.959 |
| Cardiac function index (mean ± SD) | ||||
| Myoglobin, ng/mL | 239.12±264.82 | 341.06±340.00 | 334.14±355.98 | 0.595 |
| Hypersensitive troponin I, ng/mL | 0.16±0.14 | 0.61±1.51 | 0.80±2.82 | 0.646 |
| B-type natriuretic peptide, pg/mL | 940.52±640.94 | 1,494.22±1,298.25 | 1,308.97±1,290.02 | 0.404 |
| GCS score (mean ± SD) | 11.73±2.22 | 9.95±2.68 | 10.49±3.87 | 0.314 |
| SOFA score (mean ± SD) | 11.07±4.37 | 11.91±3.95 | 12.31±3.79 | 0.512 |
| APACHE-II score (mean ± SD) | 28.27±11.13 | 30.59±7.21 | 30.24±10.17 | 0.745 |
SD, standard deviation; GM-SF, Granulocyte Macrophage-Colony-Stimulating Factor; WBC, white blood cells; RBC, red blood cells; HGB, hemoglobin; PLT, platelets; ALT, alanine aminotransferase; TBIL, total bilirubin; CRP, C-reactive protein; GCS, Glasgow score; SOFA, Sequential Organ Failure Assessment; APACHE-II, Acute Physiology and Chronic Health Evaluation II.
There was no significant difference in the tumor treatment among the 3 groups (P=0.125). Only 1 case (0.82%) was treated with targeted drugs alone, 66 cases (54.10%) were treated with chemotherapy alone, and 55 cases (45.08%) were treated with multimodality therapy. In the total population, there was 1 case (0.82%) without myelosuppression, 2 cases (1.64%) with grade I myelosuppression, 11 cases (9.01%) with grade II myelosuppression, 12 cases with grade III myelosuppression (9.84%), and 96 cases (78.69%) with IV grade myelosuppression (P=0.565). There was no significant difference in the use of blood products (P=0.581) or the use of colony-stimulating factor (P=0.165) during hospitalization.
The infection foci of the septic shock patients with tumors came from lung infections in 109 cases (89.34%), abdominal infections in 10 cases (8.20%), and bloodstream infections in 3 cases (2.46%) (P=0.176). In the culture of body fluid samples, 10 cases (8.20%) of gram-positive bacteria were detected, 49 cases (40.16%) of gram-negative bacteria were detected, and 3 cases (2.46%) of fungi were detected. In addition, the culture of humoral specimen in 60 cases (49.18%) were not detected, and there was no statistical difference among the 3 groups in the culture of humoral specimen (P=0.128). In relation to the SOFA score (the highest score within 24 hours of admission to the ICU), there was no statistical difference between the early administration group (11.07±4.37), the late administration group (11.91±3.95), and the non-administration group (12.31±3.79) (P=0.512). In relation to the APACHE-II (the highest score within 24 hours of admission to the ICU), there was no statistical difference between the early administration group (28.27±11.13), the late administration group (30.59±7.21), and non-administration group (30.24±10.17) (P=0.745).
Twenty-eight-day mortality
Administration groups vs. non-administration group
According to the Kaplan-Meier survival curve results (see Figure 1), if the start time of dobutamine administration was not considered, the 28-day mortality rate of the administration groups was 45.9% and that of the non-administration group was 58.8%. The 28-day mortality rates of the administration groups were lower than that of the non-administration group, but the difference was not statistically significant (log-rank test: χ2=2.576, P=0.109).
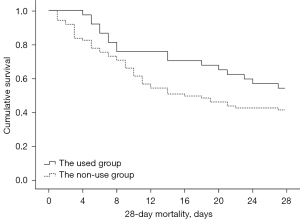
Early administration group vs. late administration group vs. non-administration group
According to the results of the Kaplan-Meier survival curve (see Figure 2), the start time of dobutamine was divided into early administration (<3 days) and late administration (≥3 days). Compared to the non-administration group, early administration (<3 days) significantly reduced the 28-day mortality rate (log-rank test: χ2=5.591, P=0.018). After comparing the groups, we found that the 28-day mortality rate (20.0%) of the early administration group was significantly lower than that of the non-administration group (58.8%), and the difference was statistically significant (HR =0.248, 95% CI: 0.077–0.796). The 28-day mortality rate of the late administration group (63.6%) was not statistically different to that of the non-administration group (58.8%) (HR =0.983, 95% CI: 0.543–1.778).
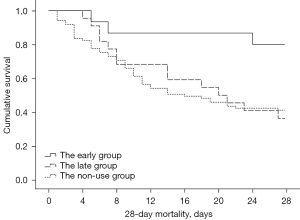
Shock reversal rate
The shock reversal rate of the early administration group was 80.0%, which was significantly higher than that of the late administration group (36.4%) and the non-administration group (41.2%), and the difference was statistically significant (P=0.014; see Table 2).
Table 2
| Characteristics | The early group (N=15) |
The late group (N=22) |
The non-use group (N=85) | P |
|---|---|---|---|---|
| 28-day mortality, n (%) | 3 (20.0) | 14 (63.6) | 50 (58.8) | 0.018 |
| Shock reversal rate, n (%) | 0.014 | |||
| Yes | 12 (80.0) | 8 (36.4) | 35 (41.2) | |
| No | 3 (20.0) | 14 (63.6) | 50 (58.8) | |
| ICU admission, days (mean ± SD) | 8.60±6.63 | 17.05±9.10 | 9.98±7.52 | <0.001 |
| The duration of mechanical ventilation, days (mean ± SD) | 5.60±6.48 | 15.09±11.26 | 8.71±13.55 | 0.049 |
SD, standard deviation; ICU, intensive care unit.
ICU length of stay
The ICU length of stay in the early administration group was 8.60±6.63 days, that of the late administration group was 17.05±9.10 days, and that of the non-administration group was 9.98±7.52 days (see Table 2). There was a statistically significant difference in the ICU length of stay among the 3 groups (P<0.001). The ICU length of stay of the early administration group was significantly lower than that of the late administration group and the non-administration group.
The duration of use of mechanical ventilation
In terms of the duration of mechanical ventilation, the duration of use of the early administration group was 5.60±6.48 days, the duration of use of the late administration group was 15.09±11.26 days, and the duration of use of the non-administration group was 8.71±13.55 days (see Table 2). The duration of mechanical ventilation of the early administration group was significantly lower than that of the other 2 groups, and there was a statistical difference (P=0.049).
Changes in heart rate and blood pressure in the administration group
- In relation to the administration of medication, initially, the average heart rate of patients was 138.73 bpm, on the 2nd day, the average heart rate was 124.89 bpm, on the 3rd day, the average heart rate was 102.95 bpm. The heart rate of patients showed a gradual downward trend as a whole (P<0.001).
- On the 1st day after the start of medication, the mean arterial pressure (MAP) was 65.86 mmHg, on the 2nd day, the MAP was 88.94 mmHg, and on the 3rd day, the MAP was 82.07 mmHg. The MAP of the patients increased continuously over 3 days (P<0.001) (Figure 3).
- On the first day of administration, the average norepinephrine dose of patients was 0.33 µg/kg/min, on day 2, the average norepinephrine dose was 0.18 µg/kg/min, and on the day 3, the average norepinephrine dose was 0.09 µg/kg/min. The norepinephrine dose of the patients showed a gradual downward trend within 3 days (P=0.058).
Discussion
Septic cardiomyopathy is a common complication of sepsis or septic shock (10). Various morbidities have been reported in the literature, with decreased myocardial contractility occurring in about 60% of patients with sepsis or septic shock (11). Sepsis-induced myocardial dysfunction is one of the main predictors of a poor prognosis in patients with sepsis (12). Mortality is 70–90% in patients with septic cardiomyopathy, and 20% in patients without septic cardiomyopathy (11,13-15). So, what is the mortality rate for cancer patients in septic shock? To assess short- and long-term mortality trends in septic shock patients with cancer by cancer type, Kim et al. examined adult cancer patients presenting to the emergency department with septic shock from 2009 to 2017, and found that among the 43,466 adult cancer patients with septic shock (90% solid and 10% hematologic cancer cases), the 30-day and 1-year mortality rates were 52.1% and 81.3%, respectively (9). The 30-day mortality rate is basically consistent with the results of our study. In our study, the overall mortality rate of tumor patients with septic cardiomyopathy was 54.92%.
Jeong et al. noted that a past history of diabetes or heart failure, a younger age, higher N-terminal pro-B-type natriuretic peptide (NT pro-BNP), and positive blood cultures are risk factors for septic cardiomyopathy (16). Unlike cardiomyopathy caused by other causes, myocardial damage caused by sepsis is usually reversible (17). Under conditions of adequate volume and arterial blood pressure, if circulatory perfusion is still insufficient in adult patients with septic shock, it is recommended that dobutamine plus norepinephrine or epinephrine alone be used (4). Conversely, the pathogenesis, diagnostic criteria, treatment of septic cardiomyopathy, and potential adverse effects of vasoactive drugs are controversial (18).
In our study, if the start time of dobutamine administration was not considered, the 28-day mortality of the medication groups was lower than that of the non-medication group, but the difference was not statistically significant. However, when comparing the start times of dobutamine administration, the early administration of the medication significantly reduced 28-day mortality (20.0%), at a rate that was much lower than that of other studies. Thus, we believe that dobutamine can provide more powerful circulatory perfusion support in the early stage of septic cardiomyopathy.
Hemodynamic tolerance to catecholamine vasopressors and inotropes is a well-established marker of mortality risk in septic shock (19-22). Kumar et al. showed that survival in patients with severe sepsis or septic shock is related to the maintenance of cardiac responsiveness to catecholamines and the improvement of cardiac function and the contractile index under dobutamine infusion (23). In our study, the early administration of medication reduced the 28-day mortality, improved the shock reversal rate, shortened the ICU stay, and decreased the duration of mechanical ventilation. Further, a prospective study by Zhou showed that in the early stage of acute respiratory distress syndrome caused by septic shock, dobutamine increased the cardiac output, reduced pulmonary edema in patients and reduced the required dose of norepinephrine (24). Fink et al. confirmed that dobutamine can improve liver function and hepatic microcirculation in septic shock (6). In a previous study, the dobutamine pretreatment group significantly improved the plasma clearance, hepatic perfusion index, and survival time of indocyanine green (6). However, through a statistical analysis of the Medical Information Mart for Intensive Care III public database, Zhu found that the in-hospital mortality rate of the dobutamine group was consistently higher than that of the non-dobutamine group (P=0.044) (25). The baseline characteristics were unbalanced between the 2 groups in this study (25). The heart rate, respiratory rate, MAP, and disease score of the medication group were higher than those of the non-medication group (P<0.05), which was the reason for the significant difference between the 2 groups [odds ratio (OR) 1.56, 95% CI: 1.01–2.40; P=0.000) (25).
Apart from dobutamine, there are 2 other types of drugs that are controversial in clinical practice (26-30). Liu et al. investigated the role of levosimendan and dobutamine in the treatment of sepsis (31). In Liu’s study, 6 randomized controlled trials (RCTs) were included, and 192 patients were selected through literature searches of multiple academic databases (31). After 24 hours of intervention, the cardiac index and left ventricular stroke work index levels of the levosimendan group combined with myocardial dysfunction were significantly better than those of the dobutamine group, and the blood lactate level was significantly lower (31). However, levosimendan had no statistically significant effect on left ventricular ejection fraction and mortality (31). Conversely, Bhattacharjee examined 7 RCTs and confirmed that levosimendan reduces the blood lactate level of patients with septic shock and increases the cardiac index of patients more than dobutamine, but found no significant differences in terms of mortality or ICU stay (32). Zhu et al. found that compared to the dobutamine group, the use of milrinone did not reduce in-hospital mortality in sepsis patients, but increased the ICU length of stay, total hospitalization time, and the rate of use of renal replacement therapy (33). In recent years, there have been some new studies on the treatment of septic cardiomyopathy, such as melatonin, to improve calcium overload (34-36), cyclosporine and its derivatives to regulate mitochondrial permeability, anti-apoptotic proteins (such as B cells leukemia protein) (37), all of which may become therapeutic targets for septic cardiomyopathy in the future.
Limitations
Our research inevitably had limitations. First, this study was a retrospective study with a small sample from a single center. Thus, the conclusions are inevitably affected by bias. Second, because the duration of dobutamine use varied among the patients in this study, only changes in heart rate and MAP at the start of the administration of the drug, 1 day after the administration of the drug, and 2 days after the administration of the drug were selected as references. After the statistical analysis, we found that the patients’ heart rates showed a gradual downward trend, the MAP showed an upward trend, and norepinephrine showed a downward trend. Finally, in this study, we did not dynamically observe the vital signs and test indicators of septic shock patients with tumors, and only selected the worst score within 24 hours of entering the ICU as a reference. Thus, related pairing studies or RCT studies need to be conducted in the future.
Conclusions
The early administration of dobutamine may reduce 28-day mortality in septic shock patients with tumor.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3226/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3226/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3226/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. SL-B2021-410-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181-247. [Crossref] [PubMed]
- Fink T, Heymann P, Taha-Melitz S, et al. Dobutamine pretreatment improves survival, liver function, and hepatic microcirculation after polymicrobial sepsis in rat. Shock 2013;40:129-35. [Crossref] [PubMed]
- Mirouse A, Vigneron C, Llitjos JF, et al. Sepsis and Cancer: An Interplay of Friends and Foes. Am J Respir Crit Care Med 2020;202:1625-35. [Crossref] [PubMed]
- Dimopoulos G, Rovina N, Patrani M, et al. Past history of stage I/II solid tumor malignancy impacts considerably on sepsis mortality: a propensity score matching analysis from the hellenic sepsis study group. BMC Infect Dis 2019;19:831. [Crossref] [PubMed]
- Kim YJ, Kim MJ, Kim YJ, et al. Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study. Cancers (Basel) 2021;13:657. [Crossref] [PubMed]
- Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol 2021;18:424-34. [Crossref] [PubMed]
- Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36:1701-6. [Crossref] [PubMed]
- Vallabhajosyula S, Shankar A, Vojjini R, et al. Impact of Right Ventricular Dysfunction on Short-term and Long-term Mortality in Sepsis: A Meta-analysis of 1,373 Patients. Chest 2021;159:2254-63. [Crossref] [PubMed]
- Ravikumar N, Sayed MA, Poonsuph CJ, et al. Septic Cardiomyopathy: From Basics to Management Choices. Curr Probl Cardiol 2021;46:100767. [Crossref] [PubMed]
- Narváez I, Canabal A, Martín C, et al. Incidence and evolution of sepsis-induced cardiomyopathy in a cohort of patients with sepsis and septic shock. Med Intensiva 2018;42:283-91. (Engl Ed). [PubMed]
- Sato R, Kuriyama A, Takada T, et al. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine (Baltimore) 2016;95:e5031. [Crossref] [PubMed]
- Jeong HS, Lee TH, Bang CH, et al. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine (Baltimore) 2018;97:e0263. [Crossref] [PubMed]
- Liu YC, Yu MM, Shou ST, et al. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front Immunol 2017;8:1021. [Crossref] [PubMed]
- Gelinas JP, Russell JA. Vasopressors During Sepsis: Selection and Targets. Clin Chest Med 2016;37:251-62. [Crossref] [PubMed]
- Allen JM, Feild C, Shoulders BR, et al. Recent Updates in the Pharmacological Management of Sepsis and Septic Shock: A Systematic Review Focused on Fluid Resuscitation, Vasopressors, and Corticosteroids. Ann Pharmacother 2019;53:385-95. [Crossref] [PubMed]
- Jozwiak M, Hamzaoui O. Adherence to surviving sepsis campaign guidelines 2016 regarding fluid resuscitation and vasopressors in the initial management of septic shock: The emerging part of the iceberg! J Crit Care 2022;68:155-6. [Crossref] [PubMed]
- Bitton E, Zimmerman S, Azevedo LCP, et al. An international survey of adherence to Surviving Sepsis Campaign Guidelines 2016 regarding fluid resuscitation and vasopressors in the initial management of septic shock. J Crit Care 2022;68:144-54. [Crossref] [PubMed]
- Colling KP, Banton KL, Beilman GJ. Vasopressors in Sepsis. Surg Infect (Larchmt) 2018;19:202-7. [Crossref] [PubMed]
- Kumar A, Schupp E, Bunnell E, et al. Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit Care 2008;12:R35. [Crossref] [PubMed]
- Zhou M, Dai J, Du M, et al. Effect of dobutamine on extravascular lung water index, ventilator function, and perfusion parameters in acute respiratory distress syndrome associated with septic shock. Artif Cells Nanomed Biotechnol 2016;44:1326-32. [Crossref] [PubMed]
- Zhu Y, Yin H, Zhang R, et al. The effect of dobutamine in sepsis: a propensity score matched analysis. BMC Infect Dis 2021;21:1151. [Crossref] [PubMed]
- Chang W, Xie JF, Xu JY, et al. Effect of levosimendan on mortality in severe sepsis and septic shock: a meta-analysis of randomised trials. BMJ Open 2018;8:e019338. [Crossref] [PubMed]
- Guo J, Zhang X, Zhu Y, et al. Comparison of dobutamine and levosimendan for treatment of sepsis-induced cardiac dysfunction: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e29092. [Crossref] [PubMed]
- Barraud D, Faivre V, Damy T, et al. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med 2007;35:1376-82. [Crossref] [PubMed]
- Sakai M, Suzuki T, Tomita K, et al. Diminished responsiveness to dobutamine as an inotrope in mice with cecal ligation and puncture-induced sepsis: attribution to phosphodiesterase 4 upregulation. Am J Physiol Heart Circ Physiol 2017;312:H1224-37. [Crossref] [PubMed]
- Sato R, Ariyoshi N, Hasegawa D, et al. Effects of Inotropes on the Mortality in Patients With Septic Shock. J Intensive Care Med 2021;36:211-9. [Crossref] [PubMed]
- Liu DH, Ning YL, Lei YY, et al. Levosimendan versus dobutamine for sepsis-induced cardiac dysfunction: a systematic review and meta-analysis. Sci Rep 2021;11:20333. [Crossref] [PubMed]
- Bhattacharjee S, Soni KD, Maitra S, et al. Levosimendan does not provide mortality benefit over dobutamine in adult patients with septic shock: A meta-analysis of randomized controlled trials. J Clin Anesth 2017;39:67-72. [Crossref] [PubMed]
- Zhu Y, Yin H, Zhang R, et al. The effect of dobutamine vs milrinone in sepsis: A big data, real-world study. Int J Clin Pract 2021;75:e14689. [Crossref] [PubMed]
- Zhang H, Liu D, Wang X, et al. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J Pineal Res 2013;55:1-6. [Crossref] [PubMed]
- Zhong J, Tan Y, Lu J, et al. Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: A novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol 2019;26:101287. [Crossref] [PubMed]
- Hu W, Deng C, Ma Z, et al. Utilizing melatonin to combat bacterial infections and septic injury. Br J Pharmacol 2017;174:754-68. [Crossref] [PubMed]
- Larche J, Lancel S, Hassoun SM, et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol 2006;48:377-85. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



