
The efficacy and safety of Zengxiao Jiandu decoction combined with definitive concurrent chemoradiotherapy for unresectable locally advanced non-small cell lung cancer: a randomized, double-blind, placebo-controlled clinical trial
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, and nearly 1/3 of patients have a locally advanced (LA) disease at the time of diagnosis (1,2). The standard of care for unresectable LA-NSCLC is definitive radiotherapy with concurrent platinum-based chemotherapy; however, it has an unsatisfactory 5-year overall survival (OS) rate of 15–25% (3,4). Due to the treatment-related toxic side effects, the low completion rate of definitive concurrent chemoradiotherapy (DCCRT) is one of the reasons for its poor overall efficacy. Thus, alleviating the toxic reactions of radiotherapy and chemotherapy and improving the completion rate of treatment are important measures in prolonging the survival time of patients with unresectable LA-NSCLC.
A large number of clinical practice and research studies have confirmed that traditional Chinese medicine (TCM) treatment regulates the balance of the human environment, alleviates the toxic reactions of radiotherapy and chemotherapy, and enhances the efficacy and improves the completion rate of radiotherapy and chemotherapy (5-8). It also enhances the body’s anti-cancer ability to prevent recurrence and metastasis, thereby prolonging the survival of patients and improving their quality of life (9). There is increasing evidence that TCM combined with radiotherapy and chemotherapy as an adjuvant therapy have a synergistic and attenuative effect, and thus is a promising model for the treatment of malignant tumors (5-9). On one hand, TCM including herbal medicine, decoction, Chinese medicine Yinpian and Chinese patent medicine has been used for more than 2000 years and has been also used in cancer treatment, safety of which is widely approved (9). On the other hand, the add-on use of TCM may improve the efficacy mainly through increasing the completion rate of radiotherapy and chemotherapy.
However, most previous clinical studies of TCM share some common limitations (10). First, there has been a lack of research on the common problems such as severe radiation pneumonitis (RP) and marrow suppression experienced during radiotherapy and chemotherapy by lung cancer patients, which may focus only on nausea, vomiting or damage of liver function. As a result, the results were decentralized and it is hard to draw a consensus. Second, there is a general lack of high-level evidence from prospective, randomized controlled studies. The majority of studies were observational studies of individualized treatment such as case reports, or intervention studies only with intermittent rather than full-process intervention. Thus, the overall credibility of these studies is not high.
In this study, we conducted a randomized, double-blind, and parallel clinical trial to evaluate the synergistic and attenuative effect of a treatment of Zengxiao Jiandu decoction combined with DCCRT in unresectable LA-NSCLC patients using epidemiological research methods. This decoction was modified Maxing Shigan decoction and used concurrently with chemoradiotherapy as an adjuvant therapy. We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2814/rc).
Methods
Study design and patients
We conducted a randomized, double-blind, placebo-controlled, multi-center phase 3 trial. Patients were eligible for inclusion in this study, if they met the following inclusion criteria: (I) had histologically confirmed stage III (IIIA, IIIB, or IIIC) NSCLC and clinically unresectable LA disease according to the 8th American Joint Committee on Cancer (AJCC) cancer staging guidelines (11,12); (II) were aged >18 years of age; (III) had adequate hematological, hepatic, renal and pulmonary function; (IV) had a World Health Organization (WHO) performance status score of 0–1; (V) had no history of any previous anti-cancer treatment except stage I cervical cancer and cutaneous basal cell carcinoma; and (VI) were expected to survive more than 6 months. Patients were excluded from this study if they met any of the following exclusion criteria: (I) had a history of lobectomy or pneumonectomy; (II) had any combination of acute or chronic diseases resulting in an intolerance to chemoradiation; (III) had an active systemic infection or an infection of the lung or pericardium; and/or (IV) was a pregnant or lactating woman.
The study was approved by Ethics Committee of Shanghai Pulmonary Hospital (No. 19228FL), and Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine has been informed and agreed with this study. The institutional review board at each center approved the study’s protocol. Written informed consent was taken from all the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was registered on the http://chictr.org.cn/ website (13).
Randomization and masking
Patients were enrolled by the physicians in charge at the Shanghai Pulmonary Hospital, Tongji University School of Medicine, and Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine. Patients were randomly assigned using a 1:1 ratio to the TCM arm or Control arm by simple randomization. Randomization was done at the Department of Radiation of Shanghai Pulmonary Hospital by computer-generated random assignment lists using a central randomization system.
Preparation of Zengxiao Jiandu decoction
The Zengxiao Jiandu decoction used modified Maxing Shigan decoction granules, consisting of 15 grams Radix glehniae, 15 grams Radix Ophiopogonis, 30 grams Astragalus root, 9 grams Radix Notoginseng, 30 grams Caulis Spatholobi, 30 grams Pyrrosia lingua, 15 grams Angelica sinensis, 15 grams Fructus lycii and 15 grams Rhizoma polygonate. The quality of the drugs involved in the treatment plan and the production of the products were in line with China’s Good Manufacturing Practice standards. The drugs used in the research were provided by the project team with TCM prescriptions and were purchased from suppliers with Chinese medicine granule production licenses. The simulated placebo granules were purchased by bidding from manufacturers with food production licenses, which require that the color, aroma, taste and appearance are consistent with the corresponding Chinese medicine granules, and indistinguishable to a third party with the naked eye. The specific ingredients were maltodextrin, caramel pigment, sunset yellow pigment, tartrazine and bitters, and modified Maxing Shigan decoction granules accounted for 10%. The Zengxiao Jiandu decoction used in this trial was a synergistic and attenuative prescription of radiotherapy and chemotherapy.
Procedures
Staging
All the patients underwent pretreatment staging, including a medical history, physical examination, routine laboratory blood tests, pulmonary function tests, and thoracoabdominal computed tomography (CT) scan. Clinical tumor-node-metastasis (TNM) staging was based on the 8th AJCC cancer staging guidelines (11,12).
Treatment
Patients with clinically unresectable LA-NSCLC were randomly assigned to receive either DCCRT (a prescribed dose of 60–66 Gy and 2 Gy per fraction that commenced on the 1st day of chemotherapy) combined with Zengxiao Jiandu decoction adjunctive therapy (the continuous oral administration of a synergistic and attenuative prescription of radiotherapy and chemotherapy twice a day from 3 days before to 7 days after radiotherapy) or placebo therapy (the continuous oral administration of placebo replacing Zengxiao Jiandu decoction twice a day from 3 days before to 7 days after radiotherapy). All the patients received concurrent chemoradiotherapy according to the National Comprehensive Cancer Network guidelines (14). As consolidation chemotherapy after DCCRT is not a standard treatment and there is no strong data to support its use, the delivery of consolidation chemotherapy by medical oncologists as per the local protocol was permitted. All the patients were treated with intensity modulated radiation therapy and platinum-based chemotherapy, either an etoposide/cisplatin (EP) or carboplatin/paclitaxel (PC) regimen. Under the EP regimen, the patients received 50 mg/m2 of etoposide on days 1–5 and 50 mg/m2 of cisplatin on days 1 and 8, every 4 weeks for 2 cycles. Under the PC regimen, the patients received 45 mg/m2 of paclitaxel and carboplatin [area under the curve (AUC) 2] on day 1 once a week.
The patients were monitored weekly to assess their toxic reactions to DCCRT according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 5.0, NCI CTCAE 5.0).
Follow-up
Patients’ responses to treatment were assessed 1 month after the end of all treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The patients were routinely followed-up every 3 months for the first 2 years after the completion of all therapy, and every 6 months for the subsequent 3 years, or earlier if clinically indicated. Each assessment included a patient history, a clinical examination, blood tests, and a thoracic CT scan. Toxicities, the pattern of first recurrence (locoregional, distant, or both), and death were recorded.
Outcomes
The primary endpoint was grade ≥3 chemoradiotherapy-related toxicities. The secondary endpoints included the completion rate of radiotherapy and chemotherapy, the clinical objective response rate (ORR), and survival. Progression-free survival (PFS) was defined as the time from randomization to the 1st event (i.e., locoregional progression, distant metastasis, or death), including the progression of disease during DCCRT treatment and the recurrence of the disease after the completion of treatment. All recurrences were evaluated by an investigator. OS was defined as the time from randomization to death. All were assessed by blinded independent central review.
Statistical analysis
To detect a grade ≥3 chemoradiotherapy-related toxicities difference of 20% after DCCRT (with a hypothesized rate of 40% for the Control arm and 20% for the TCM arm; one-sided test; alpha, 0.05; beta, 0.20), 62 analyzable patients were required for each arm. In this study, 13 events in the TCM arm and 25 events in the Control arm were expected to occur. After adjusting by 5% to account for patient ineligibility/loss, a total of 136 patients were required for each arm.
All the analyses were based on the intention-to-treat population. The Chi-square test or Fisher’s exact probability test was used to compare the categorical data. Age was compared using the Mann-Whitney test. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. A univariate Cox proportional-hazards regression model analysis was conducted to analyze the predictive role of the completion of radiotherapy and chemotherapy in survival. A logistic regression was performed for the multivariate analysis of grade ≥3 chemoradiotherapy-related toxicities and RP using a backward regression procedure, including the clinical and dosimetric factors with a P value <0.1 in the univariate analysis. The associations between the variables were tested using Pearson or Spearman correlation coefficients. Patients who were still alive at the last follow-up or lost to follow-up were regarded as missing data. As the trial is ongoing, the missing data in the analysis from the patients who were still alive at the last follow-up, will be collected in the future. Statistical significance was defined as a P value <0.05 (two-sided). All the data were analyzed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
From February 2019 to December 2020, 462 patients were assessed and 299 patients (64.7%) were excluded because they did not meet the inclusion criteria (n=234), withdrew their consent (n=49), or did not return for randomization (n=16) (see Figure 1). Study enrolment is ongoing. A total of 163 patients were randomly assigned to the TCM arm (n=82) or the Control arm (n=81). Patients and tumor characteristics were well-balanced between the 2 arms (see Table 1).
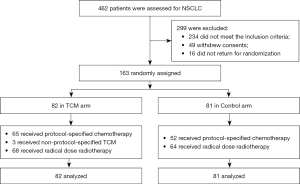
Table 1
| Characteristics | TCM (n=82), n (%) | Control (n=81), n (%) | P value |
|---|---|---|---|
| Age, years, median [range] | 64 [37–79] | 64 [34–79] | 0.830 |
| ≤60 | 25 (30.5) | 29 (35.8) | 0.471 |
| >60 | 57 (69.5) | 52 (64.2) | |
| Sex | 0.374 | ||
| Female | 11 (13.4) | 15 (18.5) | |
| Male | 71 (86.6) | 66 (81.5) | |
| Smoking history | 0.577 | ||
| Yes | 43 (52.4) | 46 (56.8) | |
| No | 39 (47.6) | 35 (43.2) | |
| Histopathology | 0.585 | ||
| Squamous cell carcinoma | 40 (48.8) | 43 (53.1) | |
| Adenocarcinoma | 33 (40.2) | 30 (37.0) | |
| Large cell carcinoma | 1 (1.2) | 3 (3.7) | |
| Unknown | 8 (9.8) | 5 (6.2) | |
| WHO performance status | 0.174 | ||
| 0 | 9 (11.0) | 15 (18.5) | |
| 1 | 73 (89.0) | 66 (81.5) | |
| TNM stage | 0.348 | ||
| IIIA | 31 (37.8) | 22 (27.2) | |
| IIIB | 41 (50.0) | 47 (58.0) | |
| IIIC | 10 (12.2) | 12 (14.8) |
Proportions were compared by Chi-square test or Fisher’s exact probability test. Age was compared using Mann-Whitney tests. TCM, traditional Chinese medicine; WHO, World Health Organization; TNM, tumor-node-metastasis.
Treatment
Of the 163 assessable patients, 132 (81.0%) received definitive-dose radiotherapy (≥60 Gy), and 117 (71.8%) received protocol-specified chemotherapy. Of the 82 patients in the TCM arm, 3 (3.6%) received non-protocol-specified TCM. The completion rates for the definitive-dose radiotherapy and protocol-specified chemotherapy were 82.9% (68/82) and 79.3% (65/82) in the TCM arm, respectively, and 79.0% (64/81) (P=0.524) and 64.2% (52/81) (P=0.033) in the Control arm, respectively (see Table 2). The overall completion rate of chemoradiotherapy was significantly higher in the TCM arm than the control arm (72.0% vs. 51.9%, P=0.008; see Table 2).
Table 2
| Variables | TCM (n=82), n (%) | Control (n=81), n (%) | P value |
|---|---|---|---|
| Treatment per protocol | 0.008 | ||
| Yes | 59 (72.0) | 42 (51.9) | |
| No | 23 (28.0) | 39 (48.1) | |
| Radiotherapy | 0.524 | ||
| ≥60 Gy | 68 (82.9) | 64 (79.0) | |
| <60 Gy | 14 (17.1) | 17 (21.0) | |
| Chemotherapy per protocol | 0.033 | ||
| Yes | 65 (79.3) | 52 (64.2) | |
| No | 17 (20.7) | 29 (35.8) | |
| Responses | |||
| Complete response | 8 (9.8) | 6 (7.4) | – |
| Partial response | 51 (62.2) | 45 (55.6) | – |
| Stable disease | 13 (15.9) | 17 (21.0) | – |
| Progressive disease | 10 (12.2) | 13 (16.0) | – |
| Objective response | 59 (72.0) | 51 (63.0) | 0.221 |
| Disease control | 72 (87.8) | 68 (84.0) | 0.480 |
Proportions were compared by Chi-square test or Fisher’s exact probability test. TCM, traditional Chinese medicine.
Clinical response to treatment
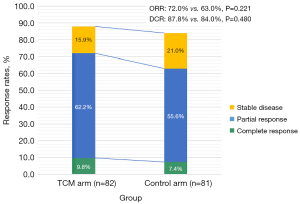
Among the 59 (72.0%) patients who achieved an objective response in the TCM arm, 8 and 51 achieved a complete response and a partial response, respectively (see Table 2). Among the 51 (63.0%) patients who achieved an objective response in the Control arm, 6 and 45 achieved a complete response and partial response, respectively (see Table 2). Zengxiao Jiandu decoction improved both the ORR (72.0% vs. 63.0%, P=0.221) and disease-control rate (DCR) (87.8% vs. 84.0%, P=0.480) (see Figure 2 and Table 2).
Survival
For the surviving patients, the median follow-up period was 24.0 months [interquartile range (IQR), 18.0–29.0 months]. Based on a data cutoff date of December 10, 2021, the median follow-up duration for the surviving patients was 24.0 months (IQR, 18.0–31.0 months) in the TCM arm and 25.0 months (IQR, 16.0–29.0 months) in the Control arm. The intention-to-treat analysis, which included the 163 assessable patients, showed that the 1- and 2-year PFS rates were significantly higher in the TCM arm than the Control arm (48.8% vs. 40.7% and 25.0% vs. 17.3%) (see Figure 3A). The median PFS period was 12.0 months (95% CI: 9.5–14.5 months) in the TCM arm and 9.0 months (95% CI: 6.4–11.7 months) [hazard ratio (HR) 0.700, log rank P=0.035] in the Control arm, respectively. The median OS period was 25.0 months (95% CI: 21.9–28.1 months) for patients in the TCM arm and 19.0 months (95% CI: 14.8–23.2 months) patients in the Control arm (HR 0.708, log rank P=0.094) (see Figure 3B). The 1- and 2-year OS rates of the TCM arm were 85.4% and 47.0%, respectively, and those of the Control arm were 71.3% and 37.7%, respectively. Further, the univariate Cox proportional-hazards regression model analysis showed that the completion of the protocol-specified chemotherapy led to a significantly longer PFS, compared with patients who failed to complete the protocol-specified chemotherapy (P=0.033). There was no significant correlation between the completion of the definitive-dose radiotherapy and PFS (P=0.137).

Treatment-related toxicities
The incidence of grade ≥3 chemoradiotherapy-related toxicities, including non-hematological and hematological toxic reactions, were higher in the TCM arm than the Control arm, but the difference was not significant (31.7% vs. 44.4%, P=0.094; see Table 3). Grade ≥3 RP occurred more frequently in the Control arm than the TCM arm (3.7% vs. 13.6%, P=0.024). Only 1 patient in the TCM arm and 2 patients in the Control arm died of grade 5 RP, respectively (1.2% vs. 2.5%, P=0.620). The frequency of grade 1–2 RP and all grades of RP was also lower in the TCM arm than the Control arm, but the difference was not statistically significant (37.8% vs. 42.0%, P=0.587; 41.5% vs. 55.6%, P=0.072). There were no significant differences in either grades 1–2 or grade ≥3 radiation esophagitis, but the incidence of grade ≥3 radiation esophagitis in the Control arm was more than twice of that in the TCM arm (14.8% vs. 7.3%, P=0.127).
Table 3
| Toxic reactions | Grade 1–2, n (%) | Grade ≥3, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TCM (n=82) | Control (n=81) | χ2 | P | TCM (n=82) | Control (n=81) | χ2 | P | ||
| Non-hematological | |||||||||
| Radiation pneumonitis | 31 (37.8) | 34 (42.0) | 0.296 | 0.587 | 3 (3.7) | 11 (13.6) | 5.109 | 0.024 | |
| Death | – | – | – | – | 1 (1.2) | 2 (2.5) | 0.352 | 0.620 | |
| Radiation esophagitis | 41 (50.0) | 44 (54.3) | 0.305 | 0.581 | 6 (7.3) | 12 (14.8) | 2.332 | 0.127 | |
| Nausea | 35 (42.7) | 30 (37.0) | 0.542 | 0.462 | 7 (8.5) | 4 (4.9) | 0.838 | 0.360 | |
| Vomiting | 29 (35.4) | 25 (30.9) | 0.373 | 0.542 | 5 (6.1) | 2 (2.5) | 1.305 | 0.253 | |
| Anorexia | 38 (46.3) | 32 (39.5) | 0.777 | 0.378 | 3 (3.7) | 2 (2.5) | 0.194 | 0.660 | |
| Fatigue | 36 (43.9) | 33 (40.7) | 0.167 | 0.683 | 4 (4.9) | 3 (3.7) | 0.137 | 0.712 | |
| Hematological | |||||||||
| Leukopenia | 41 (50.0) | 47 (58.0) | 1.056 | 0.304 | 14 (17.1) | 26 (32.1) | 4.968 | 0.026 | |
| Neutropenia | 44 (53.7) | 47 (58.0) | 0.375 | 0.575 | 8 (9.8) | 18 (22.2) | 4.723 | 0.030 | |
| Thrombocytopenia | 33 (40.2) | 45 (55.6) | 3.828 | 0.050 | 1 (1.2) | 3 (3.7) | 1.051 | 0.305 | |
| Elevated ALT | 19 (23.2) | 17 (21.0) | 0.113 | 0.737 | 1 (1.2) | 1 (1.2) | 0.000 | 0.993 | |
| Total | – | – | – | – | 26 (31.7) | 36 (44.4) | 2.805 | 0.094 | |
Proportions were compared by Chi-square test or Fisher’s exact probability test. TCM, traditional Chinese medicine; CRT, chemoradiotherapy; ALT, alanine transaminase.
The univariate analysis of the clinical and dosimetric risk factors for grade ≥3 chemoradiotherapy-related toxicities indicated that the completion of the protocol-specified chemotherapy predicted a higher incidence of g grade ≥3 chemoradiotherapy-related toxicities (P=0.022; see Table 4). The mean lung dose (MLD) was significantly associated with grade ≥3 RP (P=0.016; see Table 4). The percentage of pulmonary volume irradiated to doses exceeding 5 Gy (V5) or 20 Gy (V20) was also significantly higher in patients with grade ≥3 RP than in patients without grade ≥3 RP (P=0.017 and 0.009, respectively; see Table 4). We chose the MLD for the multivariate analysis based on the high correlation between V5, V20, and MLD and the r values from 0.884 to 0.963 as determined by the Pearson correlation analysis. The univariate and multivariate logistic regression model analysis showed that Zengxiao Jiandu decoction treatment independently indicated a decreased frequency of grade ≥3 RP (HR 0.255, 95% CI: 0.067–0.970, P=0.045), while a high MLD was identified as an independent risk factor for grade ≥3 RP (HR 1.003, 95% CI: 1.000–1.005, P=0.023; see Table 4).
Table 4
| Factor | Grade ≥3 radiochemotherapy- related toxicities |
Grade ≥3 radiation pneumonitis | |||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| P | P | Exp (B) | 95% CI | P | |||
| Treatment | |||||||
| TCM | 0.095 | 0.034 | 0.255 | 0.067–0.970 | 0.045 | ||
| Control | – | – | 1.000 | – | – | ||
| Age | 0.598 | 0.136 | – | – | – | ||
| Sex | 0.407 | 0.859 | – | – | – | ||
| ECOG | 0.608 | 0.138 | – | – | – | ||
| TNM staging | 0.237 | 0.885 | – | – | – | ||
| Radiotherapy ≥60 Gy | 0.365 | 0.346 | – | – | – | ||
| Chemotherapy per protocol | 0.022 | 0.101 | – | – | – | ||
| Smoking history | 0.804 | 0.211 | – | – | – | ||
| Histopathology | 0.418 | 0.318 | – | – | – | ||
| MLD | 0.236 | 0.016 | 1.003 | 1.000–1.005 | 0.023 | ||
| V5 | 0.506 | 0.017 | – | – | – | ||
| V20 | 0.321 | 0.009 | – | – | – | ||
| V30 | 0.495 | 0.161 | – | – | – | ||
| V50 | 0.827 | 0.519 | – | – | – | ||
| V60 | 0.535 | 0.743 | – | – | – | ||
| Dmax | 0.524 | 0.387 | – | – | – | ||
| Lung-GTV | 0.202 | 0.642 | – | – | – | ||
| PTV | 0.227 | 0.570 | – | – | – | ||
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MLD, mean lung dose; TCM, traditional Chinese medicine; GTV, gross tumor volume; PTV, planning target volume; Dmax, maximum dose; V5, the percentage lung volume irradiated to doses exceeding 5 Gy; V20, the percentage lung volume irradiated to doses exceeding 20 Gy; V30, the percentage lung volume irradiated to doses exceeding 30 Gy; V50, the percentage lung volume irradiated to doses exceeding 50 Gy; V60, the percentage lung volume irradiated to doses exceeding 60 Gy.
The frequency of grade 1–2 hematological toxicities was not significantly different between the 2 arms, except for the higher frequency of thrombocytopenia in the Control arm (40.2% vs. 55.6%, P=0.050; see Table 3). The frequency of grade ≥3 leukopenia (17.1% vs. 32.1%, P=0.026) and neutropenia (9.8% vs. 22.2%, P=0.030) were significantly lower in the TCM arm than in the Control arm. There were no significant differences in other grade ≥3 hematological toxicities. In terms of the non-hematological toxicities, Zengxiao Jiandu decoction produced a slight increase in the incidence of symptoms of the digestive tract (e.g., nausea, vomiting, anorexia and fatigue), but the difference was not statistically significant between the 2 arms. The frequencies of the other non-hematological toxicities did not differ significantly between the 2 arms.
Discussion
The results of this randomized, double-blind, placebo-controlled clinical trial have confirmed the superiority of the innovative prescription of Zengxiao Jiandu decoction combined with DCCRT over DCCRT alone in treating unresectable LA-NSCLC. The primary endpoint of the trial was unmet; however, the results showed that Zengxiao Jiandu decoction adjunctive therapy significantly reduced the occurrence of grade ≥3 RP. Additionally, the completion rate of DDCRT in the TCM arm was significantly higher than that in the Control arm, and corresponded to better PFS.
Unresectable LA-NSCLC includes unresectable phase IIIA (N2), IIIB, and IIIC NSCLC. Compared to radiotherapy alone, chemotherapy alone, and sequential chemoradiotherapy, the advantages of DCCRT in unresectable LA-NSCLC patients have been confirmed (3,4). Additionally, both induction and consolidation have failed to improve the survival of such patients (15,16). DCCRT has a curative intent; however, most patients will relapse. In clinical practice, a substantial portion of patients are incapable of completing the standard treatment due to treatment-related toxicities, which affects patients’ responses to treatment and their survival to a great extent.
Radiation-induced lung injury (RILI) is a common and clinically important complication of thoracic radiation therapy. The incidence of grade ≥2 RP, which is defined as symptomatic RILI and requires treatment, has been reported to range from 31.0% to 39.7% in various studies when combined with chemotherapy (3). Conversely, the incidence of grade ≥3 RP ranges from 4.0% to 18.4% (17,18). Once RP occurs, it is often irreversible and may lead to death, which is the main factor limiting the radiotherapy dose. Thus, numerous studies have sought to find methods to predict or prevent the occurrence of RP, especially grade ≥3 RP.
In China, TCM has been widely used in cancer treatment and is regarded as an effective adjuvant to enhance the effects and reduce the toxicities of radiotherapy and chemotherapy, but there is a lack of systematic clinical trials to verify its synergistic and attenuative effect (10). In our study, the incidence of grade ≥3 chemoradiotherapy-related toxicities were higher in the Control arm than the TCM arm, but the difference was not statistically significant. As we continue our follow-up, we may observe significant differences in the primary endpoint. Notably, the incidence of grade ≥3 RP was 13.6% in patients treated with DCCRT alone, a figure significantly higher than that observed in the TCM arm. The incidence of grades 1–2 and all grades of RP in the TCM arm was also lower than that in the Control arm, but the difference was not statistically significant.
A further analysis revealed that the MLD, V5, and V20 were also significantly associated with grade ≥3 RP, and the multivariate analysis identified Zengxiao Jiandu decoction treatment and the MLD as independent predictive factors for grade ≥3 RP, which is generally consistent with our previous research results (19). Additionally, the frequency of grade ≥3 leukopenia and neutropenia were significantly lower in the TCM arm than the Control arm. There was no significant difference in grade ≥3 thrombocytopenia and damage of liver function between the 2 arms, but this was probably because the incidence was very low in the 2 arms. Thus, our study indicates that Zengxiao Jiandu decoction significantly reduced the occurrence of severe adverse effects caused by radiotherapy and chemotherapy.
A reduction in the incidence of severe RP and marrow suppression could help to complete DCCRT, and mainly improves the completion rate of protocol-specified chemotherapy. In this study, the ORR and DCR were not significantly improved, indicating that definitive-dose radiotherapy played a major role in the elimination of primary tumors. As a result, PFS was significantly prolonged in patients who received Zengxiao Jiandu decoction treatment, who may benefit from the elimination of micro-metastases by chemotherapy. However, there was no significant difference in the OS of patients between the 2 arms. A further analysis also revealed that completion of protocol-specified chemotherapy was significantly related to PFS. Conversely, the long-term use of TCM results in very little toxicity and very few side effects (9), which we also verified in our study. Patients treated with TCM showed only slightly elevated digestive tract symptoms, including nausea, vomiting, anorexia, and fatigue. Thus, TCM had great safety while exerting a synergistic and attenuative effect, and produced almost no TCM-related toxicities.
It should be noted that this study had a number of limitations. First, the current study was an analysis with a small sample size and a short follow-up time. In the future, we will expand the sample size to confirm the reliability of the treatment scheme and gather long-term survival and safety data. Second, the specific mechanisms of the synergistic and attenuative effect are still not clear and require further exploration. Third, since the PACIFIC trial, durvalumab has been approved as a consolidation therapy following concurrent chemoradiation in unresectable LA-NSCLC (20,21). Predictably, more immune checkpoint inhibitors will be used in the consolidation therapy of unresectable stage III LA-NSCLC. The role of TCM in exerting a synergistic and attenuative effect in the age of immunotherapy needs to be further explored.
Conclusions
In conclusion, this analysis demonstrated that the Zengxiao Jiandu decoction adjunctive therapy, as compared to DCCRT alone, reduced treatment-related toxicities, improved the completion rate of DCCRT and prolonged the PFS of unresectable LA-NSCLC. The findings of the current trial support the wide use of Zengxiao Jiandu decoction in the treatment of unresectable LA-NSCLC.
Acknowledgments
Funding: The study was supported by the Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan (No. ZY[2018-2020]-FWTX-3004), Start-up Fund for Talent Introduction of Shanghai Pulmonary Hospital (grant No. 20180101), 2021 Development Fund of Discipline-Department of Radiotherapy and the National Natural Science Foundation of China (No. 1973795).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2814/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2814/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2814/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2814/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shanghai Pulmonary Hospital (No. 19228FL), and Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine has been informed and agreed with this study. Written informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Puri S, Saltos A, Perez B, et al. Locally Advanced, Unresectable Non-Small Cell Lung Cancer. Curr Oncol Rep 2020;22:31. [Crossref] [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Cheema PK, Rothenstein J, Melosky B, et al. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol 2019;26:37-42. [Crossref] [PubMed]
- Chen Y, Zhang C, Pan C, et al. Effects of Shenmai injection combined with platinum-containing first-line chemotherapy on quality of life, immune function and prognosis of patients with nonsmall cell lung cancer: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e27524. [Crossref] [PubMed]
- Dong J, Su SY, Wang MY, et al. Shenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res 2010;29:137. [Crossref] [PubMed]
- Li SG, Chen HY, Ou-Yang CS, et al. The efficacy of Chinese herbal medicine as an adjunctive therapy for advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2013;8:e57604. [Crossref] [PubMed]
- Zhang H, Zhang J, Ding H, et al. Clinical value of Tongguanteng (Radix seu Herba Marsdeniae Tenacissimae) extract combined with chemotherapy in the treatment of advanced non-small cell lung cancer: a Meta-analysis. J Tradit Chin Med 2016;36:261-70. [Crossref] [PubMed]
- Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Med 2010;76:1118-31. [Crossref] [PubMed]
- Zhao H, Zhang J, Yang F, et al. Improve the ethical review of clinical trials on traditional medicine: A cross-sectional study of clinical trial registration, ethical review, and informed consent in clinical trials of Traditional Chinese Medicine. Medicine (Baltimore) 2018;97:e13062. [Crossref] [PubMed]
- AJCC. AJCC Cancer Staging Manual, 8th edition. New York: Springer, 2016.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Chinese Clinical Trial Register (ChiCTR) [Internet]. [cited 2022 Feb 27]. Available online: https://wwwchictrorgcn/historyversionpubaspx?regno=ChiCTR2000031667
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Ahn JS, Ahn YC, Kim JH, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol 2015;33:2660-6. [Crossref] [PubMed]
- Vokes EE, Herndon JE 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007;25:1698-704. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Shepherd AF, et al. Delivering safe and effective stereotactic body radiation therapy for patients with centrally located early stage non-small cell lung cancer. Chin Clin Oncol 2020;9:39. [Crossref] [PubMed]
- Han S, Gu F, Lin G, et al. Analysis of Clinical and Dosimetric Factors Influencing Radiation-Induced Lung Injury in Patients with Lung Cancer. J Cancer 2015;6:1172-8. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


